Extreme lateral interbody fusion (XLIF) in a consecutive series of 72 patients
DOI:
https://doi.org/10.17305/bjbms.2020.5261Keywords:
Extreme lateral interbody fusion, retroperitoneal approach, spinal canal stenosis, spondylodiscitis, non-fusion, multilevel spinal surgeryAbstract
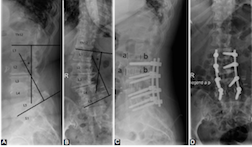
Extreme lateral interbody fusion (XLIF) has become the standard of minimally invasive lumbar segmental scoliosis treatment. Our objective is to determine the safety and efficacy of XLIF in spinal canal stenosis (SCS) and spondylodiscitis (SD). Patients treated with XLIF in our department between 2012 and 2018 were retrospectively analyzed. Patient records with clinical and radiographical parameters were evaluated. The patient cohort consists of 40 male and 32 female patients with a median age of 66.6 years. Forty-five patients had an SCS and 27 patients SD. The mean follow-up was 23 months. One level XLIF was performed in 49 patients, 2 levels in 15, 3 levels in 7 patients and 4 levels in 1 patient. All but one patient received an additional dorsal stabilization. The pain was present in all patients with a mean Visual Analogue Scale (VAS) score of 8.8 vs. postoperative VAS of 2.8 (p<0.05). Preoperative neurological deficits were found in 44 patients. Only 6 patients had a neurological deterioration, 45 patients improved, and 21 patients remained unchanged. One patient experienced a perioperative complication. Non-fusion occurred in 8 cases. There were no outcome differences regarding pain and radiological outcome between patients with SCS and SD as well as between patients with one level vs. multilevel surgery. Baseline characteristics and the radiological outcome did not differ between the two groups. Patients with SD had a higher rate of worsening of neurological deficits following surgery, a higher rate of non-fusion, and a longer hospital stay. Patients with spinal canal stenosis SCS had a longer surgery time and more frequent adjacent segment disease.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-02-05
Published 2021-10-01









