Development and validation of nomograms for predicting survival of elderly patients with stage I small-cell lung cancer
DOI:
https://doi.org/10.17305/bjbms.2020.5420Keywords:
Nomogram, small-cell lung cancer, SEER, stage I, elderlyAbstract
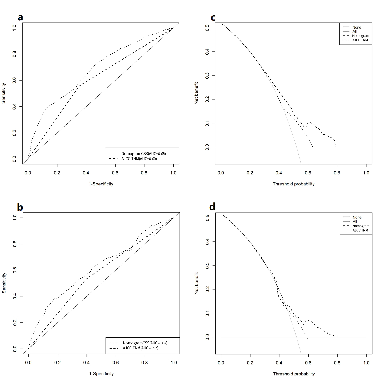
There is a lack of predictive models to determine the prognosis of elderly patients diagnosed with Stage I small-cell lung cancer (SCLC). The purpose of this study was to establish a useful nomogram to predict cancer-specific survival (CSS) in the elderly patient population. Based on the Surveillance, Epidemiology, and End Results registry database, patients aged ≥ 65 years with pathological AJCC (American Joint Committee on Cancer) Stage I SCLC from 2004 to 2014 were identified. The CSS was evaluated by the Kaplan-Meier method. Patients were randomly split into training and validation sets. In the training cohort, univariate analysis and multivariate analysis using the Cox regression identified risk factors that affected CSS, and the results were utilized to construct a nomogram for prediction of the 1-, 3-, and 5-year CSS rates of elderly patients with Stage I SCLC. The effectiveness of the nomogram was validated internally and externally by the bootstrap method. The clinical practicability and accuracy of the nomogram were evaluated by the concordance index (C-index), calibration curve, receiver operating characteristic curve, and decision curve analysis. In total, we extracted 1,623 elderly patients with Stage I SCLC. The median CSS was 34 months, and the 5-year CSS was 41%. Multivariate analysis revealed that histologic type, tumor size, age, and AJCC Stage were significant predictors of CSS. A nomogram was constructed according to the results of multivariate COX analysis. The C-indices of the nomogram for training and validation sets were 0.68 and 0.62, indicating that the nomogram demonstrated a favorable level of discrimination. The calibration curves exhibited satisfactory agreement between the actual observation and nomogram prediction. The net benefit of the nomogram was better than the AJCC TNM staging. A practical nomogram to predict the CSS of elderly patients with Stage I SCLC is constructed. The predictive tool is helpful for patient counseling and treatment decision-making.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-01-25
Published 2021-10-01









