Dishevelled family proteins (DVL1-3) expression in intrauterine growth restriction (IUGR) placentas
DOI:
https://doi.org/10.17305/bjbms.2020.5422Keywords:
intrauterine growth restriction, placenta, Dishevelled family proteins, DVL1, DVL2, DVL3Abstract
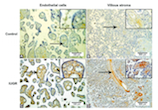
Dishevelled family proteins (DVL1, DVL2, and DVL3) are cytoplasmic proteins that are involved in canonical and non-canonical Wnt signaling pathway during embryonic development. The role of DVL proteins in the placental tissue remains mostly unknown. In the current study, we explored the role of Dishevelled proteins in naturally invasive tissue, trophoblast. Formalin-fixed paraffin-embedded samples of 15 term placentas from physiologic term pregnancies and 15 term placentas from pregnancies complicated with intrauterine growth restrictions (IUGR) were used for the study. Expression levels of mRNA for DVL1, DVL2, and DVL3 in placentas were analyzed by quantitative real-time PCR (qRTPCR). DVL1, DVL2, and DVL3 protein expression were semi-quantitatively analyzed using immunohistochemistry. The expression of DVL2 and DVL3 proteins was significantly higher in trophoblasts in placental villi from IUGR pregnancies compared with the control group of term placentas. In contrast, DVL3 protein expression was significantly higher in endothelial cells in placental villi from IUGR pregnancies compared with normal term placentas. The observed differences at protein levels between normal and IUGR placentas were not confirmed at the mRNA levels of DVL genes. Our data indicate the active involvement of DVL proteins in IUGR-related placentas. No significant changes were observed in DVL mRNA levels between the two groups of placentas. Further studies are required to explore the clinical relevance of these observations.
Citations
Downloads