Clinicopathological characteristics and survival in lung signet ring cell carcinoma: a population-based study
DOI:
https://doi.org/10.17305/bjbms.2020.5454Keywords:
Lung signet ring cell carcinoma (LSRCC), SEER, clinical characteristics, prognosisAbstract
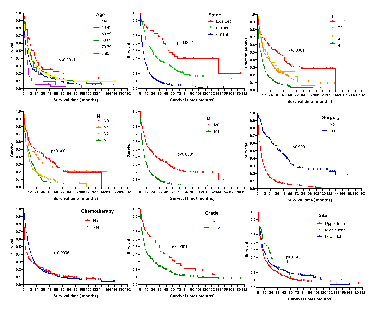
Lung signet-ring cell carcinoma (LSRCC) is a very rare type of lung cancer, the clinical characteristics, and prognosis of which remain to be clarified. In order to explore the clinicopathological and survival-related factors associated with LSRCC, we performed a large population-based cohort analysis of data included in the Surveillance, Epidemiology, and End Results (SEER) registry from 2001 to 2015. A total of 752 LSRCC and 7518 lung mucinous adenocarcinoma (LMAC) patients were incorporated into our analysis, with respective mean ages of 63.8 and 67.5 years at the time of diagnosis. LSRCC patients were significantly more likely than LMAC patients to have distant-stage disease (72.1% vs. 45.8%, p < 0.0001), tumors of a high pathological grade (40.6% vs. 10.8%, p < 0.0001), have undergone chemotherapy (62.1% vs. 39.9%, p<0.0001), be male (52.7% vs. 48.5%, p = 0.03), and be < 40 years old (3.3% vs. 1.3%, p = 0.022), whereas they were less likely to have undergone surgical treatment (52.4% vs. 77.0%, p < 0.0001). LSRCC and LMAC patients exhibited median overall survival (OS) duration of 8 and 18 months (p < 0.0001), respectively, although these differences were not significant after adjusting for confounding variables. Independent factors associated with a favorable patient prognosis included a primary site in the middle or lower lung lobe, underwent surgery, and underwent chemotherapy. However, age ≥80 years, higher grade, distant summary stage disease, and T4 stage disease were linked to poor prognosis. Patient age, tumor grade, primary tumor site, summary stage, T stage, surgery, and chemotherapy were all significantly associated with LSRCC patient prognosis.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2021-02-04
Published 2021-12-01









