The importance of speckle tracking echocardiography in the early detection of left ventricular dysfunction in patients with polycystic ovary syndrome
DOI:
https://doi.org/10.17305/bjbms.2015.552Keywords:
Polycystic ovary syndrome, speckle tracking echocardiography, subclinical left ventricular dysfunctionAbstract
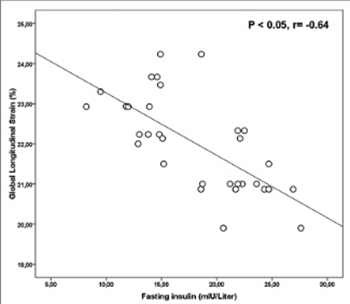
Polycystic ovary syndrome (PCOS) is characterized by hormonal and metabolic abnormalities and is thought to increase a risk for cardiovascular diseases. In this study we use speckle tracking echocardiography (STE) to evaluate left ventricular (LV) dysfunction in the early period of the disease. We enrolled 31 patients with PCOS and 32 healthy volunteers as a control group. The participants’ ages ranged between 18 and 40 years. PCOS was diagnosed according to the Rotterdam criteria. LV strain (LS) and strain rate (SR) were evaluated using apical two-chamber (2C), three-chamber (3C), and four-chamber (4C) imaging. Global LS and SR were calculated as average of three apical views. The waist-to-hip ratio, homeostasis model assessment-insulin resistance (HOMA-IR), and fasting insulin and triglyceride levels were higher in the PCOS group than in the controls (p = 0.001, p = 0.001, p = 0.001, and p = 0.005, respectively). In the PCOS group, the mitral A wave, deceleration time (DT), and isovolumetric relaxation time (IVRT) were significantly higher than in the controls (all p< 0.05). The LV global longitudinal strain (GLS) and global longitudinal SR systolic (GLSRS) were significantly lower in the PCOS patient group (both p = 0.001). There were strong negative correlations between GLS and both fasting insulin (r = −0.64) and DT (r = –0.62) (both p < 0.05). The study demonstrated that PCOS patients had decreased LV function using STE. Therefore, STE imaging appears to be useful for the early detection of subclinical LV dysfunction in patients with PCOS.
Citations
Downloads
References
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89(6):2745-9.http://dx.doi.org/10.1210/jc.2003-032046.
Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2004;18(5):671-83.http://dx.doi.org/10.1016/j.bpobgyn.2004.05.001.
Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 2002;98(1-2):33-9.http://dx.doi.org/10.1159/000064682.
Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: Pathophysiology and therapeutic implications. J Am Coll Cardiol 1993;22(1):318-25.http://dx.doi.org/10.1016/0735-1097(93)90850-Z.
Watts GF, Marwick TH. Ventricular dysfunction in early diabetic heart disease: Detection, mechanisms and significance. Clin Sci (Lond)2003;105(5):537-40.http://dx.doi.org/10.1042/CS20030211.
Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2001;86(6):2453-5.http://dx.doi.org/10.1210/jcem.86.6.7580.
Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001;103(10):1410-5.http://dx.doi.org/10.1161/01.CIR.103.10.1410.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev 1997;18(6):774-800.http://dx.doi.org/10.1210/er.18.6.774.
Bansal M, Cho GY, Chan J, Leano R, Haluska BA, Marwick TH. Feasibility and accuracy of different techniques of two-dimensional speckle based strain and validation with harmonic phase magnetic resonance imaging. J Am Soc Echocardiogr 2008;21(12):1318-25.http://dx.doi.org/10.1016/j.echo.2008.09.021.
Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: Definition of normal range. JACC Cardiovasc Imaging 2009;2(1):80-4.http://dx.doi.org/10.1016/j.jcmg.2007.12.007.
Barbosa MM, Costa Rocha MO, Vidigal DF, Bicalho Carneiro Rde C, Araújo RD, Palma MC, et al. Early detection of left ventricular contractility abnormalities by two-dimensional speckle tracking strain in Chagas’ disease. Echocardiography 2014;31(5):623-30.http://dx.doi.org/10.1111/echo.12426.
Barbosa JA, Mota CC, Simões E Silva AC, Nunes Mdo C, Barbosa MM. Assessing pre-clinical ventricular dysfunction in obese children and adolescents: The value of speckle tracking imaging. Eur Heart J Cardiovasc Imaging 2013;14(9):882-9.http://dx.doi.org/10.1093/ehjci/jes294.
Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, et al. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography 2013;30(1):88-105.http://dx.doi.org/10.1111/echo.12079.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81(1):19-25.http://dx.doi.org/10.1016/j.fertnstert.2003.10.004.
Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome- a new worldwide definition. Lancet 2005; 366:1059-62.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412-9.http://dx.doi.org/10.1007/BF00280883.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440-63.http://dx.doi.org/10.1016/j.echo.2005.10.005.
Tasolar H, Mete T, Balli M, Altun B, Çetin M, Yüce T, et al. Assessment of atrial electromechanical delay in patients with polycystic ovary syndrome in both lean and obese subjects. J Obstet Gynaecol Res 2014;40(4):1059-66.http://dx.doi.org/10.1111/jog.12308.
de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum Reprod Update 2011;17(4):495-500.http://dx.doi.org/10.1093/humupd/dmr001.
Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med 1997;126(1):32-5.http://dx.doi.org/10.7326/0003-4819-126-1-199701010-00005.
Tíras MB, Yalcìn R, Noyan V, Maral I, Yìldìrìm M, Dörtlemez O, et al. Alterations in cardiac flow parameters in patients with polycystic ovarian syndrome. Hum Reprod 1999;14(8):1949-52.http://dx.doi.org/10.1093/humrep/14.8.1949.
Marwick TH. Should we be evaluating the ventricle or the myocardium? Advances in tissue characterization. J Am Soc Echocardiogr 2004;17(2):168-72.http://dx.doi.org/10.1016/j.echo.2003.10.021.
Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 2004;17(10):1021-9.http://dx.doi.org/10.1016/j.echo.2004.06.019.
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47(4):789-93.http://dx.doi.org/10.1016/j.jacc.2005.10.040.
Matias C, Isla LP, Vasconcelos M, Almería C, Rodrigo JL, Serra V, et al. Speckle-tracking-derived strain and strain-rate analysis: A technique for the evaluation of early alterations in right ventricle systolic function in patients with systemic sclerosis and normal pulmonary artery pressure. J Cardiovasc Med (Hagerstown)2009;10(2):129-34.http://dx.doi.org/10.2459/JCM.0b013e32831af028.
Cheung YF, Liang XC, Chan GC, Wong SJ, Ha SY. Myocardial deformation in patients with Beta-thalassemia major: Aspeckle tracking echocardiographic study. Echocardiography 2010;27(3):253-9.http://dx.doi.org/10.1111/j.1540-8175.2009.01005.x.
Erdogan E, Akkaya M, Bacaksiz A, Tasal A, Turfan M, Kul S, et al. Subclinical left ventricular dysfunction in women with polycystic ovary syndrome: An observational study. Anadolu Kardiyol Derg 2013;13(8):784-90.http://dx.doi.org/10.5152/akd.2013.196.
Share BL, La Gerche A, Naughton GA, Obert P, Kemp JG. Young women with abdominal obesity have subclinical myocardial dysfunction. Can J Cardiol 2015;31(9):1195-201.http://dx.doi.org/10.1016/j.cjca.2015.02.004.
Wild RA, Grubb B, Hartz A, Van Nort JJ, Bachman W, Bartholomew M. Clinical signs of androgen excess as risk factors for coronary artery disease. Fertil Steril 1990;54(2):255-9.
Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004;110(19):3081-7.http://dx.doi.org/10.1161/01.CIR.0000147184.13872.0F.
Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, et al. Obesity cardiomyopathy: Is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr 2006;19(8):1063-71.http://dx.doi.org/10.1016/j.echo.2006.03.033.
Prelevic GM, Beljic T, Balint-Peric L, Ginsburg J. Cardiac flow velocity in women with the polycystic ovary syndrome. Clin Endocrinol (Oxf)1995;43(6):677-81.http://dx.doi.org/10.1111/j.1365-2265.1995.tb00534.x.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-07-13
Published 2015-10-19









