Chronic mechanical irritation and oral squamous cell carcinoma: A systematic review and meta-analysis
DOI:
https://doi.org/10.17305/bjbms.2021.5577Keywords:
Chronic trauma, carcinogenesis, oral squamous cell carcinoma, risk factorAbstract
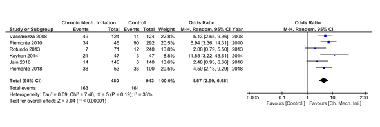
The objective of the present article was to qualitatively and quantitatively review the association between chronic mechanical irritation and oral squamous cell carcinoma (OSCC). PubMed, SCOPUS, and Web of Science databases were searched using the keyword combinations “chronic trauma and oral squamous cell carcinoma; chronic irritation and oral squamous cell carcinoma; chronic irritation and oral cancer; and chronic trauma and oral cancer.” Duplicates and irrelevant articles were excluded after the title and abstract screening. The full texts of the remaining articles were assessed using selection criteria. A total of 375 (PubMed-126; SCOPUS-152; WOS-97) articles were screened, and 343 duplicates and irrelevant articles were excluded from the study. Only 9 of the remaining 32 articles met the selection criteria and were included in the qualitative analysis. Buccal mucosa and tongue, being highly prone to chronic irritation through the dental prosthesis, were the common sites for OSCC. Edentulous subjects with ill-fitting dentures were at a high risk of developing chronic irritation associated-OSCC. According to the Joanna Briggs Institute of risk assessment, eight of the nine included studies had a low risk of bias. The quantitative analysis showed a significant association (p < 0.00001) between the chronic oral mucosal irritation and OSCC with an overall risk ratio of 2.56 at a confidence interval of 1.96-3.35. Chronic oral mucosa irritation has a significant association with OSCC, and the nature of association could be that of a potential co-factor (dependent risk factor) rather than an independent risk factor.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2021-03-28
Published 2021-12-01









