Optimization of induction of mild therapeutic hypothermia with cold saline infusion: A laboratory experiment
DOI:
https://doi.org/10.17305/bjbms.2015.565Keywords:
Cardiac arrest, intravenous infusion, therapeutic hypothermiaAbstract
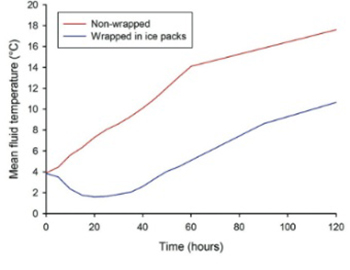
Cold fluid infusions can be used to induce mild therapeutic hypothermia after cardiac arrest. Fluid temperature higher than 4°C can increase the volume of fluid needed, prolong the induction phase of hypothermia and thus contribute to complications. We performed a laboratory experiment with two objectives. The first objective was to analyze the effect of wrapping fluid bags in ice packs on the increase of fluid temperature with time in bags exposed to ambient conditions. The second objective was to quantify the effect of insulating venous tubing and adjusting flow rate on fluid temperature increase from bag to the level of an intravenous cannula during a simulated infusion. The temperature of fluid in bags wrapped in ice packs was significantly lower compared to controls at all time points during the 120 minutes observation. The temperature increase from the bag to the level of intravenous cannula was significantly lower for insulated tubing at all infusion rates (median temperature differences between bag and intravenous cannula were: 8.9, 4.8, 4.0, and 3.1°C, for non-insulated and 5.9, 3.05, 1.1, and 0.3°C, for insulated tubing, at infusion rates 10, 30, 60, and 100 mL/minute, respectively). The results from this study could potentially be used to decrease the volume of fluid infused when inducing mild hypothermia with an infusion of cold fluids.
Citations
Downloads
References
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010. Section 4. Adult advanced life support. Resuscitation2010;81(10):1305-52.http://dx.doi.org/10.1016/j.resuscitation.2010.08.017.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med2002;346(8):557-63.http://dx.doi.org/10.1056/NEJMoa003289.
The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346(8):549-56.http://dx.doi.org/10.1056/NEJMoa012689.
Kliegel A, Janata A, Wandaller C, Uray T, Spiel A, Losert H, et al. Cold infusions alone are effective for induction of therapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation2007;73(1):46-53.http://dx.doi.org/10.1016/j.resuscitation.2006.08.023.
Isenberg DL, Pasirstein MJ. A simple method of maintaining chilled saline in the prehospital setting. Am J Emerg Med 2012;30(8):1385-8.http://dx.doi.org/10.1016/j.ajem.2011.10.007.
Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest. A randomized clinical trial. JAMA2014;311(1):45-52.http://dx.doi.org/10.1001/jama.2013.282173.
Studnek JR, Watts JA, Vandeventer S, Pearson D. Assessing the influence of insulation on intravenous fluid infusion temperature. Acad Emerg Med. 2012;19(11):1309-12.http://dx.doi.org/10.1111/acem.12006.
Mader TJ. The effect of ambient temperature on cold saline during simulated infusion to induce therapeutic hypothermia. Resuscitation2009;80(7):766-8.http://dx.doi.org/10.1016/j.resuscitation.2009.04.022.
Lee BK, Jeung KW, Lee SC, Min YI, Ryu HH, Kim MJ, et al. Augmentation of the cooling capacity of refrigerated fluid by minimizing heat gain of the fluid using a simple method of cold insulation. Acad Emerg Med2010;17(6):673-5.http://dx.doi.org/10.1111/j.1553-2712.2010.00748.x.
Larsson I, Wallin E, Rubertsson S. Cold saline infusion and ice packs alone are effective in inducing and maintaining therapeutic hypothermia after cardiac arrest. Resuscitation2010;81(1):15-9.http://dx.doi.org/10.1016/j.resuscitation.2009.09.012.
Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation2007;74(2):227-34.http://dx.doi.org/10.1016/j.resuscitation.2007.01.016.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med2009;377 Suppl:186-202.http://dx.doi.org/10.1097/CCM.0b013e3181aa5241.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-10-11
Published 2015-11-12









