The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma
DOI:
https://doi.org/10.17305/bjbms.2021.5666Keywords:
Esophageal squamous cell carcinoma, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, hemoglobin, albumin, cancer-specific survivalAbstract
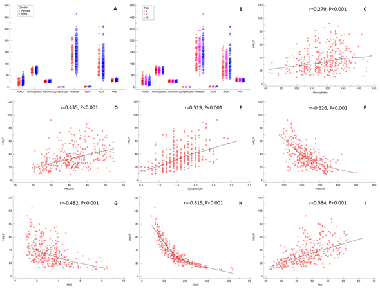
The hemoglobin, albumin, lymphocyte and platelet (HALP) score has been confirmed as a prognostic factor in several types of cancers. The current study aimed to assess the prognostic value of preoperative HALP score, an inflammatory and nutritional based score, in predicting cancer-specific survival (CSS) in resectable patients undergoing curative resection for esophageal squamous cell carcinoma (ESCC). The clinical data of 355 consecutive patients with ESCC who underwent curative resection were retrospectively conducted and analyzed. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off value for preoperative HALP. The areas under the curve (AUC) for preoperative HALP and other variables were calculated and compared. Cox regression analyses and Kaplan–Meier methods were used to identify the factors associated with CSS. According to the ROC curve, the optimal cut-off value for preoperative HALP was 31.8. The 5-year CSS for preoperative HALP low (≤31.8) and high (>31.8) was 15.1% and 47.5%, respectively (p < 0.001). Preoperative HALP had reliable abilities to predict CSS in resectable ESCC patients in any stage or gender, according to the subgroup analysis based on the patients’ cancer stage and gender. Multivariate analyses confirmed that preoperative HALP was an independent prognostic score regarding CSS in patients with resectable ESCC (p < 0.001). This study confirmed that the postoperative HALP score could be regarded as a potential independent prognostic factor for CSS in patients with resectable ESCC.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-04-09
Published 2021-12-01









