Audiological and vestibular evaluations in vitiligo patients
DOI:
https://doi.org/10.17305/bjbms.2021.5703Keywords:
VEMPs, vitiligo, vestibular system, hearing loss, otic melanocytesAbstract
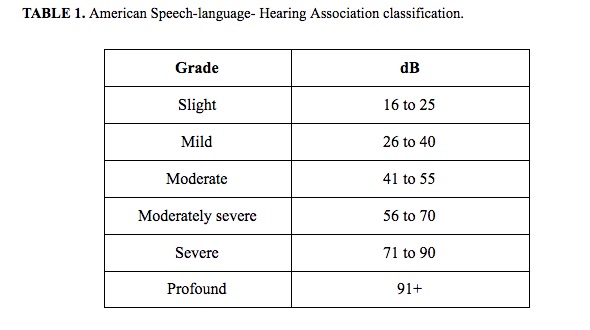
The aim of this paper was to investigate audiological abnormalities and potential vestibular injury in a sample of vitiligo subjects. Thirty-five patients with non-segmental vitiligo (NSV) were enrolled in the study. They underwent pure tonal audiometry (PTA), vestibular Fitzgerald-Hallpike caloric test, C-VEM, and O-VEMP testing. The χ2 test and multiple regression analysis were performed. At PTA, 69% of patients presented with bilateral hearing loss, 8% monaural hearing loss, and 23% normal values. Bilateral caloric stimulations were performed and demonstrated that 14% of patients had a monolateral and 9% had a bilateral pathological response. VEMPs analysis showed that 20% of patients had no O-VEMPs response and 3% had no C-VEMPs response. Comparison between the normal values of healthy subjects and NSV patients showed an alteration of VEMPs in 44%. Multiple regression showed no statistical differences. We propose a specific diagnostic protocol employing PTA, bithermal caloric tests, C-VEMP, and O-VEMP testing to evaluate audio-vestibular damage. Our data were concordant with the anatomic-physiological melanocytic distribution and their possible degeneration linked with NSV.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-07-06
Published 2022-02-01









