Multidimensional study of cell division cycle-associated proteins with prognostic value in gastric carcinoma
DOI:
https://doi.org/10.17305/bjbms.2021.5783Keywords:
Gastric cancer, cell division cycle-associated protein family, bioinformatics analysis, biomarker, prognosisAbstract
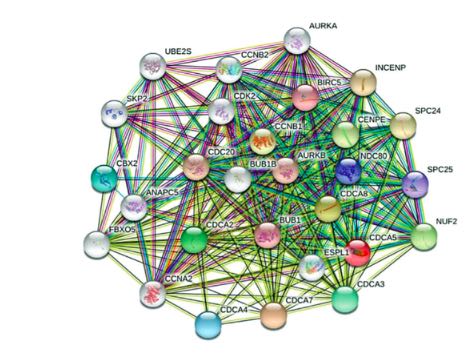
Gastric cancer (GC) represents a widespread malignancy with a poor prognosis. Hence, discovering reliable biomarkers is necessary. The cell division cycle-associated protein (CDCA) family, comprising CDCA1–8, plays a key role in tumor progression. However, whether CDCA expression has prognostic value in GC, especially stomach adenocarcinoma (STAD), has not been elucidated yet. Consequently, we conducted a multifaceted study using bioinformatic tools aimed at exploring CDCA expression levels and appraising their potential prognostic values in patients with STAD. All eight CDCAs were significantly upregulated in STAD tissues compared with healthy tissues. Elevated CDCA4/7/8 mRNA expression predicted a short overall survival, and increased CDCA7 transcriptional levels predicted a short disease-free survival. The most frequent alteration in patients with STAD was low mRNA expression. The functional enrichment analysis incorporating the gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathways showed that the cell cycle, foxO signaling pathway, and Epstein–Barr virus were relevant to the main functions of CDCAs. Finally, the immune infiltration analysis revealed a significant correlation between CDCA expression and the infiltration extent of six immunocytes. Therefore, differentially expressed CDCAs may represent potential biomarkers for the prognosis of patients with STAD that can improve survival. Furthermore, this study might offer new ideas for the design and development of immunotherapeutic drugs.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2021-05-03
Published 2022-02-01









