The prognostic significance of different proportion of signet-ring cells of colorectal carcinoma
DOI:
https://doi.org/10.17305/bjbms.2021.5856Keywords:
Rectal cancer, signet-ring cell carcinoma, prognosis, nomogramAbstract
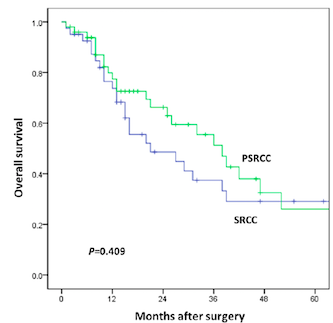
While the prognosis of patients with partial SRCC (PSRCC) has been rarely reported, colorectal signet-ring cell carcinoma (SRCC) has been associated with poor prognosis. The aim of this study was to analyze the prognosis of patients with different SRCC composition and establish a prediction model. A total of 91 patients with SRC component were included in the study. These patients were divided into two groups: SRCC group (SRC composition > 50%; n=41) and partial SRCC (PSRCC) group (SRC composition ≤ 50%; n=50). COX regression model was used to identify independent prognostic factors for overall survival (OS). A predictive nomogram was established and compared with the 7th AJCC staging system. After a median follow-up of 16 months, no significant difference in OS was observed in either group. Preoperative carcinoembryonic antigen (CEA) level, pN stage, M stage, preoperative ileus, and adjuvant chemotherapy were independent prognostic risk factors for OS (p<0.05). A nomogram for predicting the overall survival of colorectal SRCC was established with a C-index of 0.800, and it showed better performance than that of the 7th AJCC staging system (p<0.001). In summary, the ratio of SRC component was not an independent prognostic factor of the OS. Those patients with less than 50% of SRC component should be given the same clinical attention. A predictive nomogram for survival based on five independent prognostic factors was developed and showed better performance than the 7th AJCC staging system. This resulted to be helpful for individualized prognosis prediction and risk assessment.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-05-16
Published 2022-02-01









