Skeletal muscle and fiber type-specific intramyocellular lipid accumulation in obese mice
DOI:
https://doi.org/10.17305/bjbms.2021.5876Keywords:
Skeletal muscle, lipid accumulation, fiber type, obesity, insulin resistanceAbstract
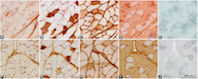
In obesity, accumulation of lipid droplets in skeletal muscle fibers and a shift towards fast muscle fiber types can both contribute to insulin resistance. However, it is not yet clear how intramyocellular lipid accumulation and fiber type changes are associated. Therefore, we investigated to what extent the lipids accumulated in a fiber type-specific manner in the functionally similar fast-, intermediate- and slow-twitch gastrocnemius, plantaris, and soleus muscles, respectively, in high-fat diet-induced obese 54-week-old female C57BL/6JOlaHsd mice (n = 9) compared to control standard-diet-treated lean mice (n = 9). A high-fat diet was administered for 26 weeks. Fiber-type specific intramyocellular lipid content analysis and muscle fiber typing were performed using histochemical analysis of lipids with Sudan Black and immunohistochemical analysis of myosin heavy chains on serial sections of skeletal muscles. Compared to the lean mice, the lipid accumulation was most prominent in types 2a and 2x/d fibers (p < 0.05) of fast-twitch gastrocnemius and intermediate plantaris muscles in the obese mice, while in slow-twitch soleus muscle, there was no significant lipid accumulation in the obese animals. Furthermore, the slow-twitch soleus muscle of the obese mice with no significant change in muscle fiber diameters exhibited the most pronounced shift towards fast-type myosin heavy chain isoform expression (p < 0.05). In contrast, the fast-twitch and intermediate-twitch gastrocnemius and plantaris muscles, respectively, in which the muscle fiber diameters increased (p < 0.05), were more resistant toward myosin heavy chain expression changes. In conclusion, we demonstrated both muscle- and fiber-type specificity in intramyocellular lipid accumulation in obese mice, suggesting that in obesity, similar muscle fiber types in different muscles accumulate lipids differentially.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-05-07
Published 2021-12-01









