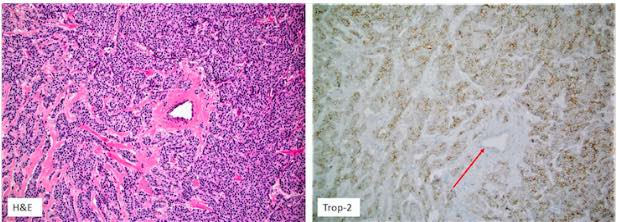
Trop-2 protein as a therapeutic target: A focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers
DOI:
https://doi.org/10.17305/bjbms.2021.6100Keywords:
Trophoblast cell-surface antigen-2, antibody-drug conjugates (ADC), Sacituzumab govitecan, breast cancer, urothelial cancer, predictive biomarkersAbstract
Antibody-drug conjugates (ADCs) represent a new class of highly potent antineoplastic drugs built by attaching a small molecule of an anticancer drug (payload) or another therapeutic agent to an antibody recognizing an epitope on the targeted cells. Trophoblast cell-surface antigen-2 (Trop-2) was originally described in trophoblasts and fetal tissues, but subsequently, its overexpression has been demonstrated in various solid malignancies. Sacituzumab govitecan, a conjugate of anti-Trop-2 antibody and SN-38 payload (an active metabolite of irinotecan), is the first in the class that has been clinically validated and approved by the Food and Drug Administration for the treatment of metastatic triple-negative breast (2020) and urothelial carcinomas (2021). In the current review, we summarize and critically appraise the most recent advances with Sacituzumab govitecan, emphasizing the predictive biomarker analysis.
Citations
Downloads