EWSR1 rearrangement in papillary thyroid microcarcinoma is related to classic morphology and the presence of small-cell phenotype
DOI:
https://doi.org/10.17305/bjbms.2021.6181Keywords:
Papillary thyroid carcinoma, small cells, EWSR1, EWSR-FLI1, carcinoma of the thyroid, Ewing family tumor elementsAbstract
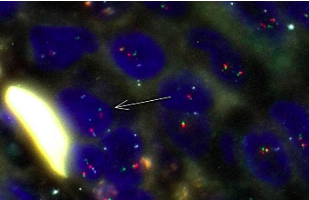
The EWSR1 rearrangements with unknown genes were detected in a high percentage of classic variants of papillary thyroid carcinoma. The small-cell carcinoma of the thyroid with Ewing family tumor elements (CEFTE) typically presents with EWSR1-FLI1 rearrangement suggesting the possible role of EWSR-FLI1 translocation in the loss of thyroid differentiation and acquisition of a small-cell phenotype. In order to determine the frequency and association of EWSR1 rearrangements, particularly the EWSR1-FLI1 fusion with clinicopathological features of papillary thyroid microcarcinoma (m-PTC) and the presence of small cells, we analyzed a series of 99 m-PTCs using the fluorescence in situ hybridization method. Ninety cases (90.9%) of m-PTC were positive for small cells. This group of m-PTC has shown more often invasive growth, lymphatics invasion, and moderate/extended intratumoral fibrosis. Three cases out of 99 were inconclusive for EWSR1 rearrangement. Eighty-nine (92.7%) and twenty-seven (28.1%) out of 96 m-PTC cases were positive for EWSR1 rearrangement and EWSR1-FLI1 fusion, respectively. m-PTC with classical architectural pattern presented more frequently with EWSR1 rearrangement relative to m-PTC with other patterns (p = 0.005). Other clinicopathological features were not related to the presence of EWSR1 rearrangement or EWSR1-FLI1 fusion. The percentage of small cells present significantly correlated with the percentage of cells positive for EWSR1-FLI1 fusion (p = 0.05) and EWSR1 rearrangement (p <0.001). EWSR1-FLI1 fusion is not rare in m-PTC and it is associated with the acquisition of small-cell phenotype. The EWSR1 gene rearrangement is a frequent event in m-PTC and is related to the classical pattern of m-PTC.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2021-07-31
Published 2022-02-01









