Effect and mechanisms of zinc supplementation in protecting against diabetic cardiomyopathy in a rat model of type 2 diabetes
DOI:
https://doi.org/10.17305/bjbms.2015.63Keywords:
diabetic cardiomyopathy, zinc, authophagy, metallothionein, oxidative stress, ratsAbstract
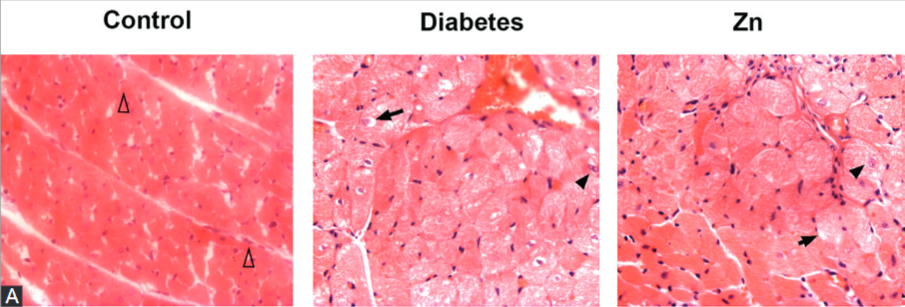
Diabetic cardiomyopathy is a prominent cause of heart failure in patients with diabetes mellitus. Currently, there is no specific treatment for diabetic cardiomyopathy. This study aimed to investigate the effect and underlying mechanisms of Zinc (Zn) supplementation in the protection against diabetic cardiomyopathy in a rat model of type 2 diabetes mellitus (T2DM). T2DM-like lesions in male Wistar rats were induced by introducing the high-fat diet and by administration of streptozocin (STZ). After STZ induction, animals with fasting plasma glucose level ≥16.7 mM were considered as diabetic, and randomly assigned to the group receiving physiological saline (control) or ZnSO4 for 56 days. On days 0, 7, 28 and 56 of treatment, animals were weighed, and their blood samples were analyzed. On day 56, hemodynamic assessment was performed right before the sacrifice of animals. Cardiac tissue specimens were collected and subjected to pathologic assessment, metallothionein (MT) concentration measurement and Western blot analysis of microtubule-associated protein light chain 3 (LC3), the marker of autophagy, and glucose-regulated protein-78 (GRP78), an oxidative stress marker. High-fat diet feeding followed by STZ administration resulted in weight loss, hyperglycemia, polydipsia, polyphagia, hemodynamic anomalies and a significant increase in the myocardial content of LC3 and GRP78 proteins, but not in MT protein. Zn supplementation effectively attenuated all these aberrations induced by high-fat diet and STZ. These findings suggest that Zn might be a protective factor in diabetic cardiomyopathy, acting in two ways: at least partially, through inhibiting autophagy and by endoplasmic reticulum stress.
Citations
Downloads
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378:31-40.
http://dx.doi.org/10.1016/S0140-6736(11)60679-X
New diabetes figures in China: IDF press statement. [Accessed 2014 July 20] Available from: http://www.idf.org/press-releases/idf-press-statement-china-study.
Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007; 115:3213-3223.
http://dx.doi.org/10.1161/CIRCULATIONAHA.106.679597
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972; 30:595-602.
http://dx.doi.org/10.1016/0002-9149(72)90595-4
Nicolino A, Longobardi G, Furgi G, Rossi M, Zoccolillo N, Ferrara N, Rengo F. Left ventricular diastolic filling in diabetes mellitus with and without hypertension. Am J Hypertens 1995; 8:382-389.
http://dx.doi.org/10.1016/0895-7061(95)00022-H
Di Bonito P, Cuomo S, Moio N, Sibilio G, Sabatini D, Quattrin S, Capaldo B. Diastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short duration. Diabet Med 1996; 13:321-324.
http://dx.doi.org/10.1002/(SICI)1096-9136(199604)13:4<321::AID-DIA3>3.0.CO;2-7
Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 2001; 24:5-10.
http://dx.doi.org/10.2337/diacare.24.1.5
Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289:194-202.
http://dx.doi.org/10.1001/jama.289.2.194
Acar E, Ural D, Bildirici U, Sahin T, Yilmaz I. Diabetic cardiomyopathy. Anadolu Kardiyol Derg 2011; 11:732-737.
Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004; 107:539-557.
http://dx.doi.org/10.1042/CS20040057
Voulgari C, Papadogiannis D, Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag 2010; 6:883-903.
http://dx.doi.org/10.2147/VHRM.S11681
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol 2012; 86:521-534.
http://dx.doi.org/10.1007/s00204-011-0775-1
Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J 2006; 27:344-350.
Oh HM, Yoon JS. Glycemic control of type 2 diabetic patients after short-term zinc supplementation. Nutr Res Pract 2008; 2:283-288.
http://dx.doi.org/10.4162/nrp.2008.2.4.283
Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol 2013; 27:137-142.
http://dx.doi.org/10.1016/j.jtemb.2012.08.001
Azevedo PS, Polegato BF, Minicucci MF, Pio SM, Silva IA, Santos PP, et al. Early echocardiographic predictors of increased left ventricular end-diastolic pressure three months after myocardial infarction in rats. Med Sci Monit 2012; 18:BR253-258.
http://dx.doi.org/10.12659/MSM.883202
Islam MS, Wilson RD. Experimentally induced rodent models of type 2 diabetes. Methods Mol Biol 2012; 933:161-174.
Akash MS, Rehman K, Chen S. Goto-Kakizaki rats: its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr Diabetes Rev 2013; 9:387-396.
http://dx.doi.org/10.2174/15733998113099990069
Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech 2009; 2:454-466.
http://dx.doi.org/10.1242/dmm.001941
Seferovic PM, Milinkovic I, Ristic AD, Seferovic Mitrovic JP, Lalic K, Jotic A, Kanjuh V, Lalic N, Maisch B. Diabetic cardiomyopathy: ongoing controversies in 2012. Herz 2012; 37:880-886.
http://dx.doi.org/10.1007/s00059-012-3720-z
Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther 2006; 111:384-399.
http://dx.doi.org/10.1016/j.pharmthera.2005.10.008
Schmidt BM, Arora R. Primary prevention of cardiovascular complications in type II diabetes patients using aspirin: a complicated tale. Am J Ther 2013; 20:275-278.
Pappachan JM, Varughese GI, Sriraman R, Arunagirinathan G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J Diabetes 2013; 4:177-189.
Ozturk N, Olgar Y, Ozdemir S. Trace elements in diabetic cardiomyopathy: An electrophysiological overview. World J Diabetes 2013; 4:92-100.
http://dx.doi.org/10.4239/wjd.v4.i4.92
Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 2006; 113:544-554.
http://dx.doi.org/10.1161/CIRCULATIONAHA.105.537894
Badole SL, Jangam GB, Chaudhari SM, Ghule AE, Zanwar AA. L-glutamine supplementation prevents the development of experimental diabetic cardiomyopathy in streptozotocin-nicotinamide induced diabetic rats. PLoS One 2014; 9:e92697.
http://dx.doi.org/10.1371/journal.pone.0092697
Daniels A, Linz D, van Bilsen M, Rutten H, Sadowski T, Ruf S, et al.. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur J Heart Fail 2012; 14:193-201.
http://dx.doi.org/10.1093/eurjhf/hfr166
Vaugelade P, Hoebler C, Bernard F, Guillon F, Lahaye M, Duee PH, et al. Non-starch polysaccharides extracted from seaweed can modulate intestinal absorption of glucose and insulin response in the pig. Reprod Nutr Dev 2000; 40:33-47.
http://dx.doi.org/10.1051/rnd:2000118
Bell SG, Vallee BL. The metallothionein/thionein system: an oxidoreductive metabolic zinc link. Chembiochem 2009; 10:55-62.
http://dx.doi.org/10.1002/cbic.200800511
Islam MS, Loots du T. Diabetes, metallothionein, and zinc interactions: a review. Biofactors 2007; 29:203-212.
http://dx.doi.org/10.1002/biof.5520290404
Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science 2004; 306:990-995.
http://dx.doi.org/10.1126/science.1099993
Quan W, Lee MS. Role of autophagy in the control of body metabolism. Endocrinol Metab (Seoul) 2013; 28:6-11.
http://dx.doi.org/10.3803/EnM.2013.28.1.6
Yamamoto S, Kazama JJ, Fukagawa M. Autophagy: a two-edged sword in diabetes mellitus. Biochem J 2013; 456:e1-3.
http://dx.doi.org/10.1042/BJ20131282
Marchetti P, Masini M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 2009; 5:1055-1056.
http://dx.doi.org/10.4161/auto.5.7.9511
Jung HS, Lee MS. Role of autophagy in diabetes and mitochondria. Ann N Y Acad Sci 2010; 1201:79-83.
http://dx.doi.org/10.1111/j.1749-6632.2010.05614.x
Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan DX, Reiter RJ. Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol Cell Endocrinol 2012; 361:12-23.
http://dx.doi.org/10.1016/j.mce.2012.04.009
Haas IG. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia 1994; 50:1012-1020.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2015 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2014-12-12
Published 2015-02-05









