Genetic predictors of the response to the treatment of hepatitis C virus infection
DOI:
https://doi.org/10.17305/bjbms.2015.632Keywords:
single nucleotide polymorphisms, interferon-lambda protein, hepatitis C chronic, treatmentAbstract
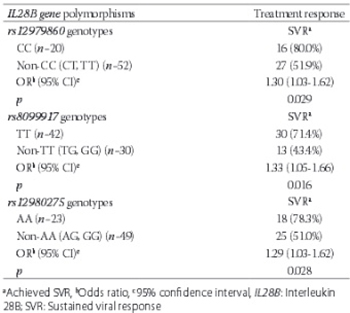
The genome-wide association studies have identified a strong association between interleukin 28B (IL28B) gene polymorphisms and the response to treatment in patients with hepatitis C virus (HCV) infection. The aim of the study was to evaluate the association between three most widely studied IL28B gene polymorphisms and the response to antiviral treatment of chronic hepatitis C. We performed the genotyping of the three IL28B gene polymorphisms: rs12979860, rs8099917, and rs12980275 in 72 Caucasian patients with chronic hepatitis C, previously treated with the combination therapy of pegylated interferon alpha (PEGIFN α) and ribavirin (RBV). The patients included in the study had finished the treatment regimen at least 6 months before enrolling in the study. We used the sustained viral response (SVR) for the evaluation of the effectiveness of the antiviral treatment, and it was tested with an assay with a sensitivity of 20 IU/mL. An SVR was achieved in 59.7% (43/72) of the treated patients. The three IL28B gene polymorphisms (CC genotype of rs12979860, TT genotype of rs8099917, and AA genotype of rs12980275) were associated with the SVR (p = 0.029, p = 0.016, and p = 0.028, respectively) in the study patients with chronic hepatitis C treated with the combination therapy of PEGIFN α and RBV. The association of IL28B gene polymorphisms with the treatment response points to the possibility of personalized medicine for the treatment of HCV infection.
Citations
Downloads
References
Wands JR. Prevention of hepatocellular carcinoma. N Engl J Med 2004;351(15):1567-70. http://dx.doi.org/10.1056/NEJMe048237.
Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, García-Álvarez M, Resino S. Meta-analysis: Implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med 2013;11:6. http://dx.doi.org/10.1186/1741-7015-11-6.
Hayes CN, Imamura M, Aikata H, Chayama K. Genetics of IL28B and HCV – Response to infection and treatment. Nat Rev Gastroenterol Hepatol 2012;9(7):406-17. http://dx.doi.org/10.1038/nrgastro.2012.101.
Glue P, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, et al. A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. The hepatitis C intervention Therapy Group. Hepatology 2000;32(4):647-53. http://dx.doi.org/10.1053/jhep.2000.16661.
Kronenberger B, Zeuzem S. Current and future treatment options for HCV. Ann Hepatol 2009;8(2):103-12.
Muir AJ, Gong L, Johnson SG, Lee MT, Williams MS, Klein TE, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon-a-based regimens. Clin Pharmacol Ther 2014;95(2):141-6. http://dx.doi.org/10.1038/clpt.2013.203.
Chayama K, Hayes CN. Interleukin-28B polymorphisms and hepatitis C virus clearance. Genome Med 2013;5(1):6. http://dx.doi.org/10.1186/gm410.
Dhingra A, Kapoor S, Alqahtani SA. Recent advances in the treatment of hepatitis C. Discov Med 2014;18(99):203-8.
Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014;384(9941):403-13. http://dx.doi.org/10.1016/S0140-6736(14)60494-3.
Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: A cure and so much more. Clin Infect Dis 2011;52(7):889-900. http://dx.doi.org/10.1093/cid/cir076.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347(13):975-82. http://dx.doi.org/10.1056/NEJMoa020047.
Booth DR, Ahlenstiel G, George J. Pharmacogenomics of hepatitis C infections: Personalizing therapy. Genome Med 2012;4(12):99. http://dx.doi.org/10.1186/gm400.
Yuen MF, Lai CL. Response to combined interferon and ribavirin is better in patients infected with hepatitis C virus genotype 6 than genotype 1 in Hong Kong. Intervirology 2006;49(1-2):96-8. http://dx.doi.org/10.1159/000087270.
Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140(5):346-55. http://dx.doi.org/10.7326/0003-4819-140-5-200403020-00010.
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461(7262):399-401. http://dx.doi.org/10.1038/nature08309.
Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009;41(10):1100-4. http://dx.doi.org/10.1038/ng.447.
Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41(10):1105-9. http://dx.doi.org/10.1038/ng.449.
Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: A genome-wide association study. Gastroenterology 2010;138(4):1338-45, 1345.e1-7.
Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology2006;131(6):1887-98. http://dx.doi.org/10.1053/j.gastro.2006.09.052.
Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, et al. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology 2012;142(4):978-88. http://dx.doi.org/10.1053/j.gastro.2011.12.055.
Lagging M, Askarieh G, Negro F, Bibert S, Söderholm J, Westin J, et al. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One 2011;6(2):e17232. http://dx.doi.org/10.1371/journal.pone.0017232.
Mangia A, Mottola L, Santoro R. Interleukin 28B polymorphisms as predictor of response in hepatitis C virus genotype 2 and 3 infected patients. World J Gastroenterol 2013;19(47):8924-8. http://dx.doi.org/10.3748/wjg.v19.i47.8924.
Tolmane I, Rozentale B, Keiss J, Ivancenko L, Subnikova N, Reinholde Z, et al. Interleukin 28B gene polymorphism and Association with chronic hepatitis C therapy results in Latvia. Hepat Res Treat 2012;2012(2):324090. http://dx.doi.org/10.1155/2012/324090.
Sticchi L, Di Biagio A, Rappazzo E, Setti M, De Rosa G, De Hoffer L, et al. Rs12979860 and rs8099917 single nucleotide polymorphisms of interleukin-28B gene: Simultaneous genotyping in Caucasian patients infected with hepatitis C virus. J Prev Med Hyg 2013;54(2):83-6.
Shaikh N, Waryah AM, Devrajani BR, Rajput MI, Hayat AS, Shaikh S. IL28B rs12980275 polymorphism shows association with response to treatment in Pakistani patients with chronic hepatitis C. J Med Virol 2015;87(5):814-20. http://dx.doi.org/10.1002/jmv.24100.
Domagalski K, Pawlowska M, Tretyn A, Halota W, Tyczyno M, Kozielewicz D, et al. Association of IL28B polymorphisms with the response to peginterferon plus ribavirin combined therapy in polish patients infected with HCV Genotype 1 and 4. Hepat Mon 2013;13(11):e13678. http://dx.doi.org/10.5812/hepatmon.13678.
Aziz H, Raza A, Ali K, Khattak JZ, Irfan J, Gill ML. Polymorphism of the IL28B gene (rs8099917, rs12979860) and virological response of Pakistani hepatitis C virus genotype 3 patients to pegylated interferon therapy. Int J Infect Dis 2015;30:91-7. http://dx.doi.org/10.1016/j.ijid.2014.09.021.
Hardy J, Singleton A. Genomewide association studies and human disease. N Engl J Med 2009;360(17):1759-68. http://dx.doi.org/10.1056/NEJMra0808700.
Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009;461(7265):798-801. http://dx.doi.org/10.1038/nature08463.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-09-09
Published 2015-11-12









