Inflammatory cells in the ascending aortic aneurysm in patients with type 2 diabetes versus patients with hypertension
DOI:
https://doi.org/10.17305/bjbms.2021.6488Keywords:
Type 2 diabetes (DM2), arterial hypertension, ascending aortic aneurysm, inflammatory cells, pro-inflammatory macrophages, anti-inflammatory macrophagesAbstract
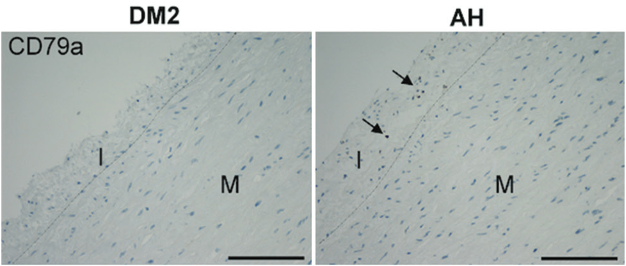
Aortic aneurysms occur relatively frequently in the ascending thoracic aorta, but are rarely seen in patients with type 2 diabetes. Our aim was to evaluate inflammatory cell infiltration in the ascending aortic aneurysm wall in patients with diabetes without arterial hypertension (DM2 group, N=6) versus hypertensive non-diabetic patients (AH group, N=34). For histologic analysis, the sections were stained with hematoxylin-eosin and Movat pentachrome. The immunohistochemical staining was used to analyze the infiltration of pro-inflammatory (CD68) and anti-inflammatory macrophages (CD163), T helper (CD4) and T killer cells (CD8), and B (CD79a) and plasma cells (CD138) in all three layers of aneurysms of both groups. The statistical significance of the differences between groups was evaluated by ANOVA and the Welch test.
In comparison to the AH group, the DM2 group developed less severe infiltration of pro-inflammatory macrophages (P=0.004) and B cells (P=0.025) in the tunica intima, and tunica media (P=0.049, P=0.007, respectively), and fewer plasma cells in the tunica media (P=0.024) and tunica adventitia (P=0.017). We found no significant differences in the number of T helper, T killer cells, and anti-inflammatory macrophages and in the amount of collagen and elastic fibers, ground substance, and smooth muscle cells in all three layers of the vessel wall. Except in tunica adventitia of DM2 group, there were more collagen fibers overall (P=0.025).
Thus, we conclude that the histological structure of the aneurysm in diabetics without hypertension is almost the same as in hypertensive patients without diabetes. Diabetics had significantly less inflammatory infiltration in all three layers of the vessel wall, and more collagen fibers in tunica adventitia.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2021 Aleksandra Milutinović, Ruda Zorc-Pleskovič

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2021-10-01
Published 2022-04-01









