A HALP score-based prediction model for survival of patients with the upper tract urothelial carcinoma undergoing radical nephroureterectomy
DOI:
https://doi.org/10.17305/bjbms.2021.6543Keywords:
postoperative survival, HALP, upper tract urothelial carcinoma, prognostic modelAbstract
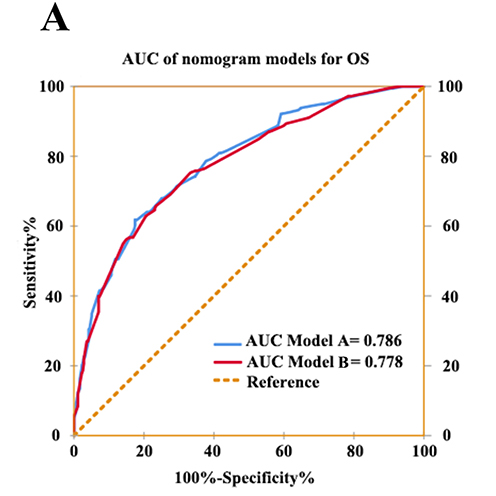
The combination of hemoglobin, albumin, lymphocyte, and platelet (HALP) score has been confirmed as an important risk biomarker in several cancers. Hence, we aimed at evaluating the prognostic value of the HALP score in patients with non-metastatic upper tract urothelial carcinoma (UTUC). We retrospectively enrolled 533 of the 640 patients from two centers (315 and 325 patients, respectively) who underwent radical nephroureterectomy (RNU) for UTUC in this study. The cutoff value of HALP was determined using the Youden index by performing receiver operating characteristic (ROC) curve analysis. The relationship between postoperative survival outcomes and preoperative HALP level was assessed using Kaplan-Meier analysis and Cox regression analysis. As a result, the cutoff value of HALP was 28.67 and patients were then divided into HALP<28.67 group and HALP≥28.67 group. Kaplan-Meier analysis and log-rank test revealed that HALP was significantly associated with overall survival (OS) (P<0.001) and progression-free survival (PFS) (P<0.001). Multivariate analysis demonstrated that lower HALP score was an independent risk factor for OS (HR=1.54, 95%CI, 1.14-2.01, P=0.006) and PFS (HR=1.44, 95%CI, 1.07-1.93, P=0.020). Nomograms of OS and PFS incorporated with HALP score were more accurate in predicting prognosis than without. In the subgroup analysis, the HALP score could also stratify patients with respect to survival under different pathologic T stages. Therefore, pretreatment HALP score was an independent prognostic factor of OS and PFS in UTUC patients undergoing RNU.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-12-17
Published 2022-04-01









