Obstructive sleep apnoea patients vs laryngopharyngeal reflux disease: Non-invasive evaluation with NBI and pepsin detection in tears
DOI:
https://doi.org/10.17305/bjbms.2021.6712Keywords:
Obstructive sleep apnea, laryngopharyngeal reflux disease, Narrow Band Imaging, pepsinAbstract
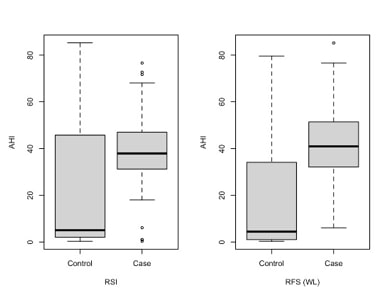
Obstructive sleep apnoea (OSA) and laryngopharyngeal reflux disease (LPR) are two common diseases that lower patients' quality of life. OSA is defined by cyclic events of airflow obstruction that occur during sleep, while LPR is characterized by upper airway inflammatory signs and symptoms due to the return of gastroduodenal gaseous and liquid elements. pH-metry is the gold standard in LPR diagnosis, but considering its invasiveness among other negative traits, questionnaires that catalog symptoms and signs of the disease such as Reflux Symptoms Index (RSI) and Reflux Finding Score (RFS) are preferred. Moreover, LPR can be evaluated by testing the presence of pepsin in tears, and Narrow Band Imaging (NBI) has been introduced for the early diagnosis of larynx oncological disease. This paper aims to test whether LPR is more frequent in OSA patients than in control ones, performing a non-invasive protocol composed of RSI, RFS test (with light vs. NBI techniques), followed by pepsin detection in tears. 68 LPR patients were enrolled in the study (45 with OSA and 23 without OSA). A strong linear relationship between Apnea-Hypopnea Index (AHI) and Oxygen Desaturation Index (ODI) was found, and patients who presented pepsin in tears had higher values of AHI and ODI in comparison to patients without it. Pathological RFS and NBI showed higher values of AHI and ODI in comparison to the control group. Furthermore, pathological RSI showed higher values of AHI and ODI in comparison to the control group. In conclusion, this diagnostic combined non-invasive protocol may be a good method to perform an early diagnosis of LPR.
Citations
Downloads
Downloads
Additional Files
Published
License
Copyright (c) 2022 Annalisa Pace, Valeria Rossetti, Alessandro Milani, Giannicola Iannella, Salvatore Cocuzza, Antonino Maniaci, Danilo Alunni Fegatelli, Annarita Vestri, Antonio Greco, Marco de Vincentiis, Francesca Giovannetti, Rocco Plateroti, Giuseppe Magliulo

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2021-12-24
Published 2022-07-29









