Tumor protein P63 Regulated 1 contributes to inflammation and cell proliferation of cystitis glandularis through regulating the NF-кB/cyclooxygenase-2/prostaglandin E2 axis
DOI:
https://doi.org/10.17305/bjbms.2021.6763Keywords:
TPRG1, inflammation, Proliferation, cystitis glandularis, NF-кB, cyclooxygenase 2, prostaglandin E2, COX2, PGE2Abstract
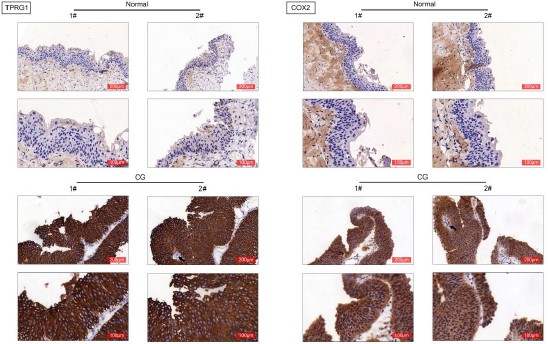
Cystitis glandularis is characterized by chronic inflammation and hyperproliferation of bladder mucosa, and contributes to progression of bladder adenocarcinoma. TPRG1 (Tumor Protein P63 Regulated 1) is related to cellular inflammatory response, and dysregulation of TPRG1 in tumor tissues is associated with tumor early recurrence. The effect of TPRG1 on cystitis glandularis was investigated in this study. Firstly, bladder specimen were isolated from patients with cystitis glandularis and E. coli-induced cystitis rat. Expression of TPRG1 was found to be up-regulated in the bladder specimen. Moreover, adeno-associated virus (AAV)-mediated silence of TPRG1 was delivered into rat, and data from hematoxylin and eosin (H and E) staining showed that injection with AAV-shTPRG1 ameliorated E. coli-induced histological changes in bladder tissues of rats, and suppressed the inflammatory response. Secondly, TPRG1 was also increased in primary cystitis glandularis cells. Knockdown of TPRG1 decreased cell proliferation of primary cystitis glandularis cells, and suppressed the migration. Thirdly, cyclooxygenase-2 (COX-2) was up-regulated in the bladder specimen isolated from patients with cystitis glandularis and E. coli-induced cystitis rat. Injection with AAV-shTPRG1 reduced protein expression of COX-2, p65 and prostaglandin E2 (PGE2) in the bladder specimen. Lastly, interference of COX-2 attenuated TPRG1 over-expression-induced increase of cell proliferation and migration in the primary cystitis glandularis cells. In conclusion, TPRG1 promoted inflammation and cell proliferation of cystitis glandularis through activation of NF-кB/COX2/PGE2 axis.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2021-12-17
Published 2022-02-01









