G-protein-coupled estrogen receptor-30 gene polymorphisms are associated with uterine leiomyoma risk
DOI:
https://doi.org/10.17305/bjbms.2016.683Keywords:
uterine leiomyoma, GPR30 (GPER1), gene, polymorphism, estrogen receptorAbstract
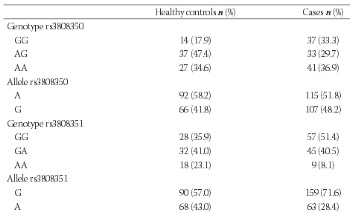
The G-protein-coupled estrogen receptor (GPR30, GPER-1) is a member of the G-protein-coupled receptor 1 family and is expressed significantly in uterine leiomyomas. To understand the relationship between GPR30 single nucleotide polymorphisms and the risk of leiomyoma, we measured the follicle-stimulating hormone (FSH) and estradiol (E2) levels of 78 perimenopausal healthy women and 111 perimenopausal women with leiomyomas. The participants’ leiomyoma number and volume were recorded. DNA was extracted from whole blood with a GeneJET Genomic DNA Purification Kit. An amplification-refractory mutation system polymerase chain reaction approach was used for genotyping of the GPR30 gene (rs3808350, rs3808351, and rs11544331). The differences in genotype and allele frequencies between the leiomyoma and control groups were calculated using the chi-square (χ2) and Fischer’s exact test. The median FSH level was higher in controls (63 vs. 10 IU/L, p=0.000), whereas the median E2 level was higher in the leiomyoma group (84 vs. 9.1 pg/mL, p=0.000). The G allele of rs3808351 and the GG genotype of both the rs3808350 and rs3808351 polymorphisms and the GGC haplotype increased the risk of developing leiomyoma. There was no significant difference in genotype frequencies or leiomyoma volume. However, the GG genotype of the GPR30 rs3808351 polymorphism and G allele of the GPR30 rs3808351 polymorphism were associated with the risk of having a single leiomyoma. Our results suggest that the presence of the GG genotype of the GPR30 rs3808351 polymorphism and the G allele of the GPR30 rs3808351 polymorphism affect the characteristics and development of leiomyomas in the Turkish population.
Citations
Downloads
References
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107.
http://dx.doi.org/10.1067/mob.2003.99
Ekin M, Cengiz H, Öztürk E, Kaya C, Yasar L, Savan K. Genitourinary symptoms and their effects on quality of life in women with uterine myomas. Int Urogynecol J. 2014;25(6):807-810
http://dx.doi.org/10.1007/s00192-013-2295-4
Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037-1054.
http://dx.doi.org/10.1289/ehp.5787
Grandien K, Berkenstam A, Gustafsson JA. The estrogen receptor gene: promoter organization and expression. Int J Biochem Cell Biol. 1997;29(12):1343-1369
http://dx.doi.org/10.1016/S1357-2725(97)89967-0
Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996-2009.
http://dx.doi.org/10.1210/me.2005-0525
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625-1630.
http://dx.doi.org/10.1126/science.1106943
Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D,et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149(10):4846-4856.
http://dx.doi.org/10.1210/en.2008-0269
Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285-308.
http://dx.doi.org/10.1146/annurev.physiol.67.040403.115914
Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5(6):421-427.
http://dx.doi.org/10.1038/nchembio.168
Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, et al. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196(4):386.e1-9; discussion 386.e9-11.
Huang GS, Gunter MJ, Arend RC, Li M, Arias-Pulido H, Prossnitz ER, et al. Co-expression of GPR30 and ERbeta and their association with disease progression in uterine carcinosarcoma. Am J Obstet Gynecol. 2010;203(3):242.e1-5.
http://dx.doi.org/10.1016/j.ajog.2010.04.046
Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci. 2012;19(7):684-693.
http://dx.doi.org/10.1177/1933719111431000
Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, et al. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12(21):6359-6366.
http://dx.doi.org/10.1158/1078-0432.CCR-06-0860
He YY, Cai B, Yang YX, Liu XL, Wan XP. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009;100(6):1051-1061.
http://dx.doi.org/10.1111/j.1349-7006.2009.01148.x
Tica AA, Dun EC, Tica OS, Gao X, Arterburn JB, Brailoiu GC, et al. G protein-coupled estrogen receptor 1-mediated effects in the rat myometrium. Am J Physiol Cell Physiol. 2011;301(5):C1262-1269.
http://dx.doi.org/10.1152/ajpcell.00501.2010
Welsh T, Johnson M, Yi L, Tan H, Rahman R, Merlino A, et al. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERα activates ERK1/2 signaling in term myometrium. J Endocrinol. 2012;212(2):227-238.
http://dx.doi.org/10.1530/JOE-11-0358
Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Virchows Arch. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. 2008;453(6):557-569.
Jakimiuk AJ, Bogusiewicz M, Tarkowski R, Dziduch P, Adamiak A, Wróbel A, et al. Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal women. Fertil Steril. 2004;82:1244-1249.
http://dx.doi.org/10.1016/j.fertnstert.2004.02.130
Tian R, Wang Z, Shi Z, Li D, Wang Y, Zhu Y, et al. Differential expression of G-protein-coupled estrogen receptor-30 in human myometrial and uterine leiomyoma smooth muscle. Fertil Steril. 2013;99(1):256-263.
http://dx.doi.org/10.1016/j.fertnstert.2012.09.011
Giess M, Lattrich C, Springwald A, Goerse R, Ortmann O, Treeck O. GPR30 gene polymorphisms are associated with progesterone receptor status and histopathological characteristics of breast cancer patients. J Steroid Biochem Mol Biol. 2010;118(1-2):7-12.
http://dx.doi.org/10.1016/j.jsbmb.2009.09.001
Andersen J. Growth factors and cytokines in uterine leiomyomas. Semin Reprod Endocrinol. 1996;14(3):269-282.
http://dx.doi.org/10.1055/s-2007-1016336
Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20(8):409-416.
http://dx.doi.org/10.1016/j.tem.2009.04.006
Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, et al. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49(6):1358-1363.
http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.089995
Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, et al. GPR30 predicts poor survival for ovarian cancer. Oncol. 2009;114(3):465-471.
http://dx.doi.org/10.1016/j.ygyno.2009.05.015
Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Andò S, et al. Endocrinology. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. 2008;149(10):5043-5051.
Kleuser B, Malek D, Gust R, Pertz HH, Potteck H. 17-Beta-estradiol inhibits transforming growth factor-beta signaling and function in breast cancer cells via activation of extracellular signal-regulated kinase through the G protein-coupled receptor 30. Mol Pharmacol. 2008;74(6):1533-1543.
http://dx.doi.org/10.1124/mol.108.046854
Chevalier N, Paul-Bellon R, Camparo P, Michiels JF, Chevallier D, Fénichel P. Genetic variants of GPER/GPR30, a novel estrogen-related G protein receptor, are associated with human seminoma. Int J Mol Sci. 2014;15(1):1574-1589.
http://dx.doi.org/10.3390/ijms15011574
Korkmaz HA, Edgünlü T, Eren E, Demir K, Çakir ED, Çelik SK, et al. GPR30 Gene Polymorphisms Are Associated with Gynecomastia Risk in Adolescents. Horm Res Paediatr. 2015;83(3):177-182.
http://dx.doi.org/10.1159/000369013
Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83(6):1875-1880.
http://dx.doi.org/10.1210/jc.83.6.1875
Luoto R, Kaprio J, Rutanen EM, Taipale P, Perola M, Koskenvuo M. Heritability and risk factors of uterine fibroids--the Finnish Twin Cohort study. Maturitas. 2000;37(1):15-26.
http://dx.doi.org/10.1016/S0378-5122(00)00160-2
Cha PC, Takahashi A, Hosono N, Low SK, Kamatani N, Kubo M, et al. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat Genet. 2011;43(5):447-450.
http://dx.doi.org/10.1038/ng.805
Edwards TL, Hartmann KE, Velez Edwards DR. Variants in BET1L and TNRC6B associate with increasing fibroid volume and fibroid type among European Americans. Hum Genet. 2013;132(12):1361-1369.
http://dx.doi.org/10.1007/s00439-013-1340-1
Aissani B, Zhang K, Wiener H.Follow-up to genome-wide linkage and admixture mapping studies implicates components of the extracellular matrix in susceptibility to and size of uterine fibroids. Fertil Steril. 2015;103(2):528-534.e13.
http://dx.doi.org/10.1016/j.fertnstert.2014.10.025
Morosova EB, Chukhlovin AB, Kulagina NV, Kipich NV, Totolian AA. Functional gene polymorphism of matrix metalloproteinase-1 is associated with benign hyperplasia of myo- and endometrium in the Russian population. Genet Test Mol Biomarkers. 2012;16(9):1032-1037.
http://dx.doi.org/10.1089/gtmb.2011.0376
Attar R, Atasoy H, İnal-Gültekin G, Timirci-Kahraman Ö, Güleç-Yilmaz S, Dalan AB, et al. The effects of PON1 gene Q192R variant on the development of uterine leiomyoma in Turkish patients. In Vivo. 2015;29(2):243-246.
Gultekin GI, Yilmaz SG, Kahraman OT, Atasoy H, Dalan AB, Attar R, et al. Lack of influence of the ACE1 gene I/D polymorphism on the formation and growth of benign uterine leiomyoma in Turkish patients. Asian Pac J Cancer Prev. 2015;16(3):1123-1127.
http://dx.doi.org/10.7314/APJCP.2015.16.3.1123
Shen Y, Ren ML, Xu J, Xu Q, Ding YQ, Wu ZC, et al. A multicenter case-control study on screening of single nucleotide polymorphisms in estrogen-metabolizing enzymes and susceptibility to uterine leiomyoma in han chinese. Gynecol Obstet Invest. 2014;77(4):224-230.
http://dx.doi.org/10.1159/000360083
Ates O, Demirturk F, Toprak M, Sezer S. Polymorphism of catechol-o-methyltransferase and uterine leiomyoma. Mol Cell Biochem. 2013;375(1-2):179-183.
Alleyne AT, Austin S, Williams A. Distribution of CYP17α polymorphism and selected physiochemical factors of uterine leiomyoma in Barbados. Meta Gene. 2014; 9(2):358-365.
Downloads
Additional Files
Published
How to Cite
Accepted 2015-10-12
Published 2016-01-06









