Serum leptin, bone mineral density and the healing of long bone fractures in men with spinal cord injury
DOI:
https://doi.org/10.17305/bjbms.2015.693Keywords:
leptin, long bones fracture, spinal cord injury, bone mineral densityAbstract
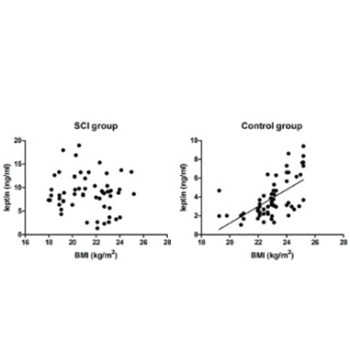
Previously reported fracture rates in patients with spinal cord injury range from 1% to 20%. However, the exact role of spinal cord injury in bone metabolism has not yet been clarified. In order to investigate the effects of serum leptin and bone mineral density on the healing of long bone fractures in men with spinal cord injury, 15 male SCI patients and 15 matched controls were involved in our study. The outcome indicated that at 4 and 8 weeks after bone fracture, callus production in patients with spinal cord injury was lower than that in controls. Besides, bone mineral density was significantly reduced at 2, 4 and 8 weeks. In addition, it was found that at each time point, patients with spinal cord injury had significantly higher serum leptin levels than controls and no association was found between serum leptin level and bone mineral density of lumbar vertebrae. Moreover, bone mineral density was positively correlated with bone formation in both of the groups. These findings suggest that in early phases i.e. week 4 and 8, fracture healing was impaired in patients with spinal cord injury and that various factors participated in the complicated healing process, such as hormonal and mechanical factors.
Citations
Downloads
References
Sugi MT, Davidovitch R, Montero N, Nobel T, Egol KA. Treatment of lower-extremity long-bone fractures in active, nonambulatory, wheelchair-bound patients. Orthopedics 2012;35(9):e1376-1382.
http://dx.doi.org/10.3928/01477447-20120822-25
Bertoni L, Ferretti M, Cavani F, Zavatti M, Resca E, Benelli A et al. Leptin increases growth of primary ossification centers in fetal mice.J Anat 2009;215(5):577-583.
http://dx.doi.org/10.1111/j.1469-7580.2009.01134.x
Kume K, Satomura K, Nishisho S, Kitaoka E, Yamanouchi K, Tobiume S et al. Potential role of leptin in endochondral ossification.J Histochem Cytochem 2002;50(2):159-169.
http://dx.doi.org/10.1177/002215540205000204
Gaspar AP, Brandão CM, Lazaretti-Castro M.Bone mass and hormone analysis in patients with spinal cord injury: evidence for a gonadal axis disruption.J Clin Endocrinol Metab 2014;99(12):4649-4655.
http://dx.doi.org/10.1210/jc.2014-2165
Wang L, Tang X, Zhang H, Yuan J, Ding H, Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J Spinal Cord Med 2011;34(5):501-509.
http://dx.doi.org/10.1179/2045772311Y.0000000034
Haidari F, Mohammadshahi M, Borsi SH, Haghighizadeh MH, Malgard S.Comparison of essential fatty acid intakes and serum levels of inflammatory factors between asthmatic and healthy adults: a case- control study.Iran J Allergy Asthma Immunol 2014;13(5):335-42.
Thomas T.The complex effects of leptin on bone metabolism through multiple pathways.Curr Opin Pharmacol 2004;4(3):295-300.
http://dx.doi.org/10.1016/j.coph.2004.01.009
Rodrigues L, Mouta R, Costa AR, Pereira A, Capela e Silva F, Amado F et al. Effects of high-fat diet on salivary α-amylase, serum parameters and food consumption in rats. Arch Oral Biol 2015;60(6):854-862.
http://dx.doi.org/10.1016/j.archoralbio.2015.02.015
Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015;64(1):105-113.
http://dx.doi.org/10.1016/j.metabol.2014.10.021
Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T et al. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone 2005;37(5):607-621.
http://dx.doi.org/10.1016/j.bone.2005.05.009
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL et al.Leptin regulates bone formation via the sympathetic nervous system. Cell 2002;111(3):305-317.
http://dx.doi.org/10.1016/S0092-8674(02)01049-8
Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 2007;40(5):1209-1216.
http://dx.doi.org/10.1016/j.bone.2007.01.006
Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 1999;140(4):1630-1638.
http://dx.doi.org/10.1210/en.140.4.1630
Liu BQ, Gong X, Jin Z. Effect of Danzhi decoction on expression of angiogenesis factors in patients with sequelae of pelvic inflammatory disease. Asian Pac J Trop Med 2014;7(12):985-990.
http://dx.doi.org/10.1016/S1995-7645(14)60173-5
Gordeladze JO, Drevon CA, Syversen U, Reseland JE.Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 2002;85(4):825-836.
http://dx.doi.org/10.1002/jcb.10156
Aro H, Eerola E, Aho AJ, Penttinen R. Healing of experimental fractures in the denervated limbs of the rat. Clin Orthop Relat Res 1981;(155):211-217.
http://dx.doi.org/10.1097/00003086-198103000-00034
Aro H, Eerola E, Aho AJ.Fracture healing in paraplegic rats. Acta Orthop Scand 1985;56(3):228-32.
http://dx.doi.org/10.3109/17453678508993001
Jiang SD, Jiang LS, Dai LY. Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif Tissue Int 2007;80(3):167-175.
http://dx.doi.org/10.1007/s00223-006-0085-4
Ding WG, Jiang SD, Zhang YH, Jiang LS, Dai LY.Bone loss and impaired fracture healing in spinal cord injured mice.Osteoporos Int 2011;22(2):507-515.
http://dx.doi.org/10.1007/s00198-010-1256-8
Frotzler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippuner K. Long-bone fractures in persons with spinal cord injury. Spinal Cord 2015;53(9):701-704.
http://dx.doi.org/10.1038/sc.2015.74
Lane JM, Sandhu HS.Current approaches to experimental bone grafting.Orthop Clin North Am 1987;18(2):213-225.
Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, Hjertner O, et al. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res 2001;16(8):1426-1433.
http://dx.doi.org/10.1359/jbmr.2001.16.8.1426
Hamrick MW, Ferrari SL.Leptin and the sympathetic connection of fat to bone.Osteoporos Int 2008;19(7):905-912
http://dx.doi.org/10.1007/s00198-007-0487-9
Wilcockson DC, Campbell SJ, Anthony DC, Perry VH. The systemic and local acute phase response following acute brain injury. J Cereb Blood Flow Metab 2002;22(3):318-326.
http://dx.doi.org/10.1097/00004647-200203000-00009
Miell JP, Englaro P, Blum WF. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res 1996;28(12):704-707.
http://dx.doi.org/10.1055/s-2007-979882
Morimoto Y, Conroy SM, Ollberding NJ, Kim Y, Lim U, Cooney RV et al. Ethnic differences in serum adipokine and C-reactive protein levels: the multiethnic cohort.Int J Obes (Lond) 2014;38(11):1416-1422.
http://dx.doi.org/10.1038/ijo.2014.25
Osuna JA, Gómez-Pérez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men.Arch Androl 2006;52(5):355-361.
http://dx.doi.org/10.1080/01485010600692017
Sámano R, Martínez-Rojano H, Rodríguez-Ventura AL, Godínez-Martínez E, Tolentino M, López-de-Cárdenas G et al. Bone biomarkers and its relation with bone mineral density in adults and adolescents during the first year postpartum. Arch Latinoam Nutr 2014;64(1):24-33.
Pop LC, Sukumar D, Tomaino K, Schlussel Y, Schneider SH, Gordon CL et al.Moderate weight loss in obese and overweight men preserves bone quality.Am J Clin Nutr 2015;101(3):659-667.
http://dx.doi.org/10.3945/ajcn.114.088534
Reid IR. Relationships among body mass, its components, and bone. Bone 2002;31(5):547-555.
http://dx.doi.org/10.1016/S8756-3282(02)00864-5
Vasilkova O, Mokhort T, Sharshakova T, Hayashida N, Takamura N. Leptin is an independent determinant of bone mineral density in men with type 2 diabetes mellitus. Acta Diabetol 2011;48(4):291-295.
http://dx.doi.org/10.1007/s00592-011-0266-0
Zhong N, Wu XP, Xu ZR, Wang AH, Luo XH, Cao XZ et al. Relationship of serum leptin with age, body weight, body mass index, and bone mineral density in healthy mainland Chinese women. Clin Chim Acta 2005;351(1-2):161-168.
http://dx.doi.org/10.1016/j.cccn.2004.09.003
Sato M, Takeda N, Sarui H, Takami R, Takami K, Hayashi M et al. Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab 2001; 86(11):5273-5276.
http://dx.doi.org/10.1210/jcem.86.11.8020
Ushiroyama T, Ikeda A, Hosotani T, Higashiyama T, Ueki M. Inverse correlation between serum leptin concentration and vertebral bone density in postmenopausal women. Gynecol Endocrinol 2003;17(1):31-36.
http://dx.doi.org/10.1080/gye.17.1.31.36
Yilmazi M, Keleş I, Aydin G, Orkun S, Bayram M, Sevinc FC et al. Plasma leptin concentrations in postmenopausal women with osteoporosis. Endocr Res 2005;31(2):133-138.
http://dx.doi.org/10.1080/07435800500229276
Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R.Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone 2001;28(1):113-117.
http://dx.doi.org/10.1016/S8756-3282(00)00408-7
Sabour H, Norouzi Javidan A, Latifi S, Shidfar F, Vafa MR et al.Relationship between leptin and adiponectin concentrations in plasma and femoral and spinal bone mineral density in spinal cord-injured individuals. Spine J 2015; 15(1):1-9.
http://dx.doi.org/10.1016/j.spinee.2014.06.009
Doherty AL, Battaglino RA, Donovan J, Gagnon D, Lazzari AA, Garshick E et al. Adiponectin is a candidate biomarker of lower extremity bone density in men with chronic spinal cord injury. J Bone Miner Res 2014;29(1):251-259.
http://dx.doi.org/10.1002/jbmr.2020
Ragnarsson KT. Bone loss and fractures in limbs paralyzed by spinal cord injury: Prevention, diagnosis, and treatment. J Spinal Cord Med 2015;38(1):10-12.
http://dx.doi.org/10.1179/2045772314Y.0000000200
Coupaud S, McLean AN, Purcell M, Fraser MH, Allan DB. Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone 2015;74:69-75.
http://dx.doi.org/10.1016/j.bone.2015.01.005
Wölfl C, Schweppenhäuser D, Gühring T, Takur C, Höner B, Kneser U et al. Characteristics of bone turnover in the long bone metaphysis fractured patients with normal or low Bone Mineral Density (BMD). PLoS One 2014;9(5):e96058.
http://dx.doi.org/10.1371/journal.pone.0096058
Paillard T.Exercise and bone mineral density in old subjects: theorical and practical implications.Geriatr Psychol Neuropsychiatr Vieil 2014;12(3):267-273.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-10-17
Published 2015-11-16









