First-line treatment of patients with HER2-positive metastatic gastric and gastroesophageal junction cancer
DOI:
https://doi.org/10.17305/bjbms.2021.7069Keywords:
CF, FOLFOX, gastric cancer, gastroesophageal junction cancer, trastuzumabAbstract
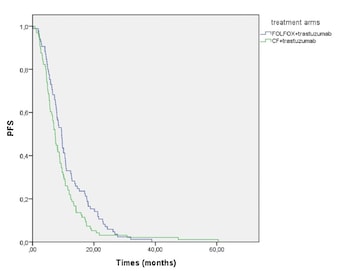
Fluoropyrimidine+cisplatin/oxaliplatin+trastuzumab therapy is recommended for the first-line treatment of HER2-positive metastatic gastric adenocarcinoma. However, there is no comprehensive study on which platinum-based treatment should be preferred. This study aimed to compare the treatment response and survival characteristics of patients with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) cancer who received fluorouracil, oxaliplatin, and leucovorin (mFOLFOX)+trastuzumab or cisplatin and fluorouracil (CF)+trastuzumab as first-line therapy. It was a multicenter, retrospective study of the Turkish Oncology Group, which included 243 patients from 21 oncology centers. There were 113 patients in the mFOLFOX+trastuzumab arm and 130 patients in the CF+trastuzumab arm. The median age was 62 years in the mFOLFOX+trastuzumab arm and 61 years in the CF+trastuzumab arm (P = 0.495). 81.4% of patients in the mFOLFOX+trastuzumab arm and 83.1% in the CF+trastuzumab arm had gastric tumor localization (P = 0.735). The median progression-free survival (PFS) was significantly higher in the mFOLFOX+trastuzumab arm (9.4 months vs. 7.3 months, P = 0.024). The median overall survival (OS) was similar in both groups (18.4 months vs. 15.1 months, P = 0.640). Maintenance trastuzumab was continued after chemotherapy in 101 patients. In this subgroup, the median OS was 23.3 months and the median PFS was 13.3 months. In conclusion, mFOLFOX+trastuzumab is similar to CF+trastuzumab in terms of the median OS, but it is more effective in terms of the median PFS in the first-line treatment of HER2-positive metastatic gastric and GEJ cancer. The choice of treatment should be made by considering the prominent toxicity findings of the chemotherapy regimens.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Selin Aktürk Esen, Yakup Ergun, Cihan Erol, Rukiye Arikan, Muhammed Muhiddin Er, Muhammed Mustafa Atci, Atakan Topçu, Gökhan Uçar, Baran Akagündüz, Musa Bariş Aykan, Miraç Özen, Naziyet Köse Baytemur, Melike Özçelik, Elif Şahin , Denizcan Güven , Serkan Menekşe, Naziye Ak, Fatih Teker, Engin Kut, Teoman Şakalar, Özkan Alan, Turgut Kaçan , Nazim Serdar Turhal, Saadettin Kiliçkap, Sema Türker , Mehmet Ali Nahit Şendur, Osman Köstek, Mustafa Karaağaç , Abdullah Sakin , Haci Mehmet Türk, Dilek Çağlayan , Şener Cihan, Yusuf Açikgöz , Doğan Uncu

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-04-04
Published 2022-09-16









