Acceptance, effects, and tolerability in the vaccination process against SARS-CoV-2 among cancer patients in Bosnia and Herzegovina: A single-center cross-sectional study
DOI:
https://doi.org/10.17305/bjbms.2021.7134Keywords:
COVID-19, vaccination, cancer patients, acceptanceAbstract
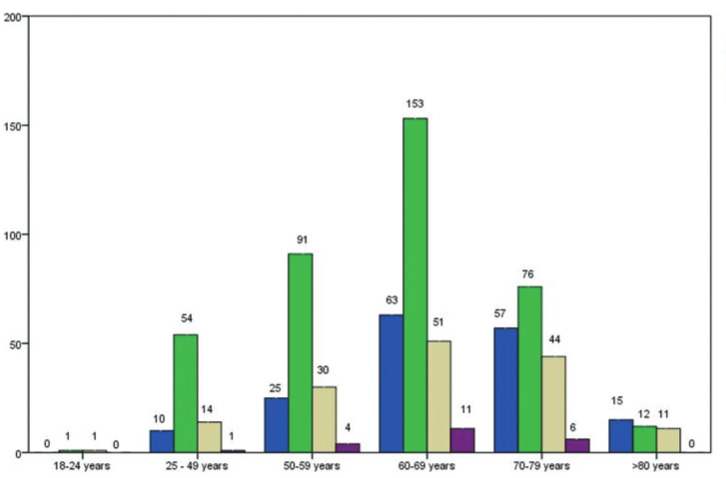
The SARS-CoV-2 pandemic has been the main public health issue since the end of 2019. The vaccination campaign in Bosnia and Herzegovina started in April 2021, with several vaccines available. Our study aimed to evaluate the acceptance, effects, and tolerability of vaccines against SARS-COV-2 among cancer patients. We conducted a cross-sectional, observational study between 22 October and 30 November 2021, at the Clinic of Oncology, Clinical Center University of Sarajevo. Patients were enrolled during their regular visit to the Clinic of Oncology by agreeing to completean individual paper questionnaire. The study included 1063 patients with malignant diseases, of whom 681 (64.1%) were adequately vaccinated patients. In the study population, 76.9% of patients reported that they did not experience any side effects due to vaccination, while only 0.5% had side effects, causing a delay in their treatment. Among adequately vaccinated patients, there were 40 patients (3.8%) who were infected with SARS-CoV-2 after the second or booster dose of the vaccine. Five patients (0.5%) were hospitalized due to COVID-19 after being adequately vaccinated. The findings of our study suggest that cancer patients have a higher acceptance of vaccines against SARS-CoV-2 than the general population in Bosnia and Herzegovina. Vaccination side effects are tolerable and do not cause major delays in specific cancer treatment. The protective effects of COVID-19 vaccines in the cancer patients presented in our study are comparable to available results of similar studies, which included the general population.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Timur Ceric, Emir Sokolović, Anes Pašić, Emina Borovac-Gurda, Velda Smajlbegović, Berisa Hasanbegović, Emina Bičakčić Filipović, Elma Kapisazović, Selma Sokolović, Semir Bešlija

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-04-02
Published 2022-09-16









