Risk of graft loss in kidney transplant recipients after aortic valve replacement
DOI:
https://doi.org/10.17305/bjbms.2022.7720Keywords:
Aortic valve stenosis, aortic valve replacement, TAVI, kidney transplant recipients, graft survivalAbstract
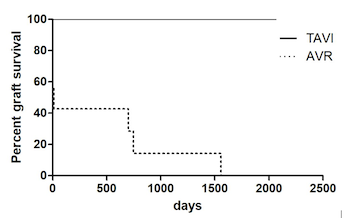
Surgical aortic valve replacement (SAVR) in kidney transplant recipients (KTR) is associated with high morbidity and mortality, and an increased risk of postoperative graft failure potentially leading to graft loss. Transcatheter aortic valve implantation (TAVI) emerged as an alternative in high-risk patients. However, data on TAVI in kidney transplant recipients are limited. We performed a retrospective analysis of 40 KTR in which aortic valve replacement was performed at our center between 2005 and 2015. The outcomes and follow-up of TAVI (n=20; 2010-2015) and SAVR (n=20; 2005-2015) were analyzed with respect to patient and graft survival. Baseline characteristics in both groups were comparable. Hospital stay after TAVI was significantly shorter compared to SAVR (19 [11.5-21.75] days vs. 33 [21-62] days, p=0.001). Acute graft failure occurred more frequently after SAVR (45% vs. 89.5%; p=0.006). Thirty-day mortality was 10% in both groups. However, in-hospital mortality reached 25% in the SAVR group (TAVI 10%), indicating a more complicated course after surgery. Moreover, during a median follow-up time of 1928 days in TAVI patients and 2717 days in patients after SAVR, graft loss occurred only in the surgically treated group (n=7). While one-year survival after TAVR was 90% compared to 69% after SAVR, long-term follow-up showed comparable results (at 5 years: TAVI 58% vs. 52% SAVR; log-rank-test: p=0.86). In KTR, TAVI can be performed with good mid- to long-term results. Compared to SAVR, renal outcomes seem to be improved after TAVI, suggesting better graft survival.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Stefan Büttner, Carolin Zöller, Sammy Patyna, Anisa Gradascevic, Helge Weiler, Mark Rosenberg, Thomas Walther, Andreas M. Zeiher, Helmut Geiger, Mariuca Vasa-Nicotera, Ingeborg Hauser, Stephan Fichtlscherer

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-07-11
Published 2023-01-06









