Transfusion transmitted virus and dengue virus among healthy blood donors: A prevalence report from Jordan
DOI:
https://doi.org/10.17305/bjbms.2022.7832Keywords:
Transfusion transmitted virus, dengue virus, prevalence, infection, JordanAbstract
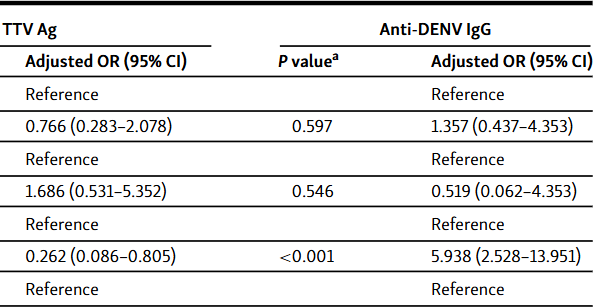
Transfusion transmitted virus (TTV) is thought to contribute to non-A non-E hepatitis and other diseases. Dengue virus (DENV) is a serious mosquito-borne pathogen. Reports on TTV and DENV in Jordan and the Middle East and North Africa region are limited. Herein, the prevalence of TTV antigen and anti-DENV IgG antibodies among apparently healthy blood donors from Northern Jordan and the Northern Agwar region of Jordan was investigated using an enzyme-linked immunosorbent assay. Chi-square test and binary logistic regression were used to correlate positivity with possible infection risk factors (age, sex, residence location, and occupation). One hundred ninety apparently healthy blood donors were included in the study (age 18 - 54 years). TTV antigen was detected in 17.9% of the samples. Lower antigen positivity was observed among Agwar residents than non-residents (7.1% vs 24.5%; chi-square test P < 0.001), which was confirmed by regression analysis (odds ratio 0.262 [95% confidence interval 0.086-0.805]; P = 0.019). Antigen positivity did not differ by age, sex, or occupation. Seropositivity for anti-DENV IgG was 17.9%. Seropositivity did not differ by age, sex, or occupation. Higher seropositivity was observed among Agwar residents than non-residents (36.1% vs 9.4%; chi-square test P < 0.001), which was confirmed by regression analysis (odds ratio 5.420 [95% confidence interval 2.377-12.359]; P < 0.001). Overall, low TTV antigen prevalence and DENV seroprevalence were found among blood donors from Northern Jordan and the Northern Agwar region of Jordan.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Samer Swedan, Doaa Al-saleh

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-12-01
Published 2023-05-01









