Intensity modulated radiation therapy for elderly patients with esophageal cancer: Our experience
DOI:
https://doi.org/10.17305/bjbms.2022.7835Keywords:
Esophageal cancer, elderly, intensity modulated radiotherapy, treatment mode, prognosisAbstract
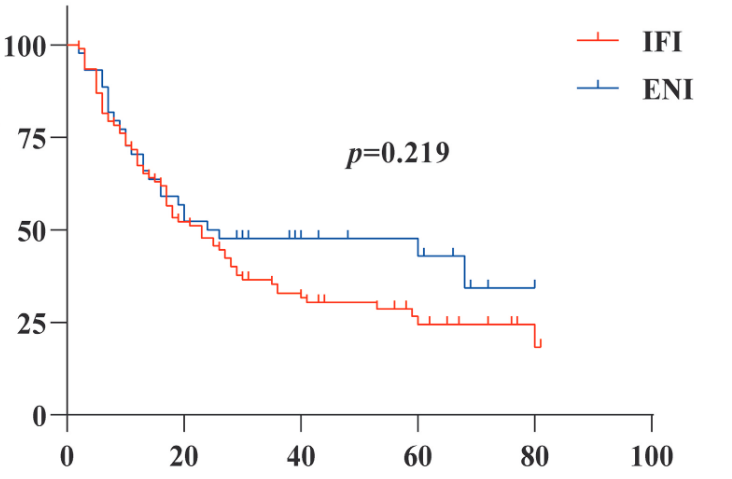
The aim of this study was to discuss the treatment mode of radical radiotherapy for elderly patients with esophageal cancer. The clinical data of 136 elderly patients (≥60 years old) with esophageal cancer (EC) who received radical intensity-modulated radiotherapy in the Second Affiliated Hospital of Xi'an Jiaotong University from January 2015 to December 2019 were retrospectively analyzed. Cox risk model was used for multivariate prognostic analysis, and Kaplan Meier method was used to calculate progression free survival (PFS) and overall survival (OS). Cox regression analysis showed that ECOG score, basic diseases, T stage, radiation dose, radiation injury and chemotherapy were the prognostic factors of elderly patients. The median OS of the radiotherapy group, concurrent chemoradiotherapy group and sequential chemoradiotherapy group were 17, 41 and 10 months (p=0.009), respectively. The 3-year OS and PFS of concurrent intravenous chemotherapy and oral chemotherapy were 50%, 42.9% and 34.1%, 28.6% (p=0.641, p=0.702), respectively. The median OS of IFI and ENI were 23 and 24 months (p=0.219) and the local recurrence rate were 59.8% and 43.2% (p=0.069), respectively, but the incidence and mortality of radiation pneumonia and esophagitis in ENI were higher. The 3-year OS and PFS the low-dose group (≤60Gy) and high-dose group (>60Gy) were 19.1%, 40.4% and 14.9%, 29.2% (p=0.012, p=0.049), respectively. In conclusion, for elderly patients with inoperable EC, radical chemoradiotherapy should be considered a preferable selection. Among them, oral drugs and high-dose involved field irradiation exhibited better curative effects and safety.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Dan Li, Xiaoxiao Liu, Yuchen Wang, Yingying Jin, Fang Li, Hongbing Ma

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-08-14
Published 2023-03-16









