Assessment of clinical utility and predictive potential of pre-chemotherapy soluble urokinase plasminogen activator receptor: Observational single center study
DOI:
https://doi.org/10.17305/bjbms.2022.7857Keywords:
Cancer, biomarkers, plasminogen activator receptor, urokinase type, progression-free survival, hemostasisAbstract
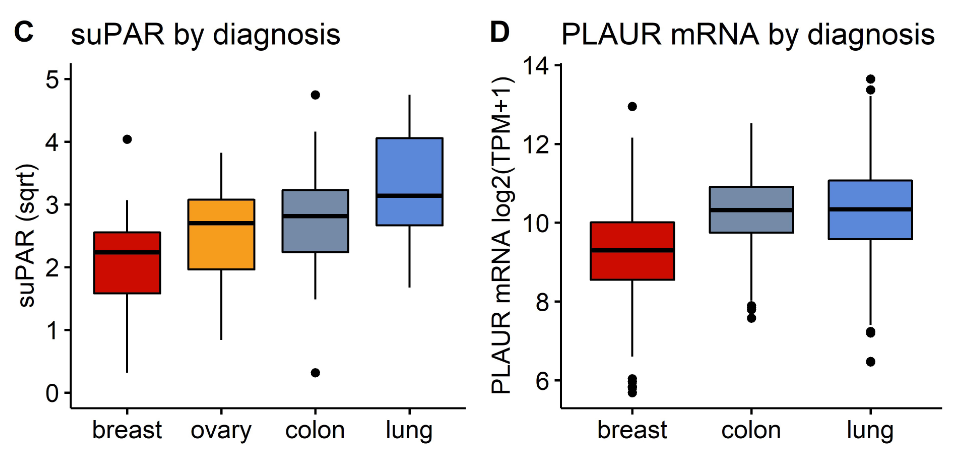
Alteration of urokinase plasminogen activator receptor (uPAR) in neoplasms is a prerequisite for invasiveness and metastatic ability. In the present study, we aimed to evaluate the relationship of pre-chemotherapy soluble uPAR (suPAR) with the odds for metastasis, lack of disease control, and its predictive ability for progression-free survival (PFS). Baseline plasma suPAR levels were measured by ELISA in 89 patients with various cancers prior to initiation of systemic treatment. Patients were followed prospectively until metastatic progression or death. TCGA Pan-Cancer dataset was mined for available RNAseq expression data of the PLAUR gene in patients with breast, colon, and lung cancer, and therelevant genomic and clinical data were extracted for further analysis. Pre-chemotherapy suPAR levels were significantly associated with white blood cell counts and fibrinogen and were significantly elevated both in patients with metastatic disease and in patients with progression. Increasing suPAR was significantly associated with odds for progression in the prespecified multivariate analysis (odds ratio 2.47, 95% confidence interval 1.3 – 5.11). In univariate Cox regression, suPAR was predictive of shortened progression-free survival (PFS) (hazard ratio 1.065, 95% confidence interval 1.002 – 1.13; p = 0.041). There was a trend towards shortened PFS in patients with higher baseline suPAR levels (cutoff 8.1 ng/mL). In the TCGA lung cancer cohort, PLAUR mRNA expression was significantly associated with shortened PFS in both univariate and multivariate analyses. High PLAUR gene expression conferred significant survival disadvantage only in patients with colon and lung cancer. SuPAR may bear predictive potential for adverse outcomes in cancer, but its utility as a biomarker seems to be more pronounced in cancers with associated inflammatory state.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Elina Beleva, Snezhana Stoencheva, Tanya Deneva, Ivanka Nenova, Zhanet Grudeva-Popova

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-09-02
Published 2023-03-16









