Cytotoxic T-lymphocyte-associated protein 4 +49A/G polymorphism in Down syndrome children with Hashimoto’s thyroiditis
DOI:
https://doi.org/10.17305/bb.2022.7869Keywords:
Down syndrome, Hashimoto’s thyroiditis, CTLA-4 49A/GAbstract
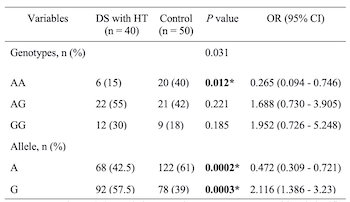
Thyroid dysfunction is the most common endocrine disorder in Down syndrome (DS) children. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is one of the immune regulatory genes that correlates with Hashimoto’s thyroiditis (HT). However, studies on CTLA-4 +49A/G in DS children with HT are still limited. We aimed to evaluate CTLA-4 +49A/G gene polymorphism in DS children with HT. This case-control study, conducted from February 2020 to February 2022 at Dr. Soetomo General Hospital, Surabaya, enrolled 40 DS children with HT and 50 healthy children. The DNA sequencing was performed to identify the polymorphism (Sanger sequencing). Thyroid peroxidase antibodies (TPOAb), thyroglobulin antibodies (TgAb), thyroid-stimulating hormone (TSH), and free thyroxine (FT4) levels were analyzed by enzyme-linked immunosorbent assay (ELISA). The mean age of DS children with HT was 1.78 years. Males predominated in the study population. Subjects with GG genotype were diagnosed earliest with hypothyroidism (8 months) compared with other studies. The most common thyroid dysfunction was central hypothyroidism, with TgAb positivity present in all patients. The AA genotype (odds ratio [OR] 0.265, 95% confidence interval [CI] 0.094–0.746; P = 0.012) and A allele (OR 0.472, 95% CI 0.309–0.721; P = 0.0002) were significantly more frequent in the control group. The AG genotype (OR 2.65, 95% CI 0.094–0.746; P = 0.003) and G allele (OR 2.116, 95% CI 1.386–3.23; P = 0.003) were more frequent in the DS with HT group. The age of the subjects in this study was younger than in previous studies. The AG genotype and the G allele were more prevalent in the DS with HT group and may be a risk factor in HT development in DS children. Furthermore, the AA genotype may act as a protective factor against HT in DS children.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Muhammad Faizi, Nur Rochmah, Yuni Hisbiyah, Rayi Kurnia Perwitasari, Qurrota Ayuni Novia Putri, Sukmawati Basuki, Anang Endaryanto, Soetjipto Soetjipto

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-01-06
Published 2023-07-03









