Interleukin-37 suppresses the cytotoxicity of hepatitis B virus peptides-induced CD8+ T cells in patients with acute hepatitis B
DOI:
https://doi.org/10.17305/bjbms.2022.8260Keywords:
Hepatitis B virus, acute infection, CD8 T cells, cytotoxicity, interleukin-37Abstract
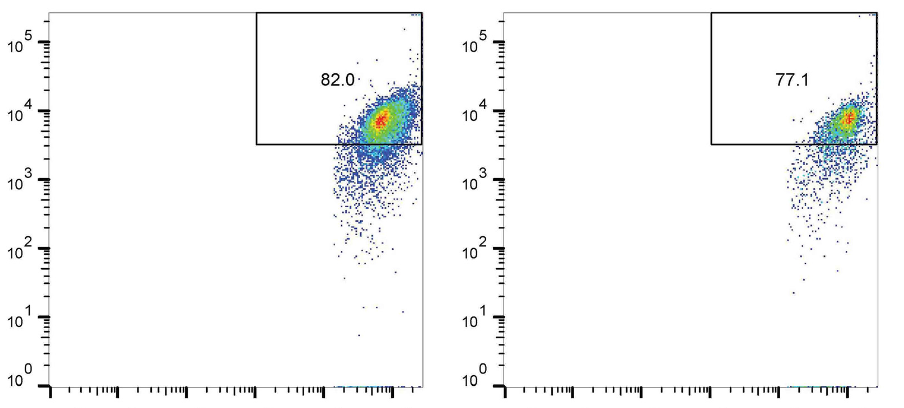
Interleukin-37 (IL-37) is a newly identified anti-inflammatory cytokine, owning immunosuppressive activity in infectious diseases. The aim of this study was to investigate the regulatory function of IL-37 on CD8+ T cells during hepatitis B virus (HBV) infection. Eighteen acute hepatitis B (AHB) patients, thirty-nine chronic hepatitis B (CHB) patients, and twenty controls were enrolled. IL-37 concentration was measured by ELISA. IL-37 receptor subunits expressions on CD8+ T cells were assessed by flow cytometry. Purified CD8+ T cells were stimulated with HBV peptides and recombinant IL-37. Perforin and granzyme B secretion was investigated by ELISPOT. Programmed death-1 (PD-1) and cytotoxic T-lymphocyte associated protein-4 (CTLA-4) mRNA expressions were semi-quantified by real-time PCR. CD8+ T cell cytotoxicity was assessed in direct contact and indirect contact coculture with HepG2.2.15 cells. Plasma IL-37 level was down-regulated and negatively correlated with aminotransferase levels in AHB patients. There were no significant differences of IL-37 receptor subunits among AHB patients, CHB patients, and controls. Exogenous IL-37 stimulation suppressed HBV peptides-induced perforin and granzyme B secretion by CD8+ T cells in AHB patients, but not in CHB patients. Exogenous IL-37 stimulation did not affect proinflammatory cytokines secretion as well as PD-1/CTLA-4 mRNA expressions in CD8+ T cells in AHB and CHB patients. Exogenous IL-37 stimulation dampened HBV peptide-induced CD8+ T cell cytotoxicity in a cell-to-cell contact manner. The current data indicated that acute HBV infection might induce down-regulation of IL-37, which might be associated with enhanced CD8+ T cell cytotoxicity and liver damage.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Qian Liu, Qiang Zhou, Mingrui Wang, Bo Pang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-10-28
Published 2023-05-01









