A prognostic nomogram for predicting overall survival in colorectal mucinous adenocarcinoma patients based on the SEER database
DOI:
https://doi.org/10.17305/bjbms.2022.8297Keywords:
Colorectal cancer, mucinous adenocarcinoma, nomogram, overall survival, SEER programAbstract
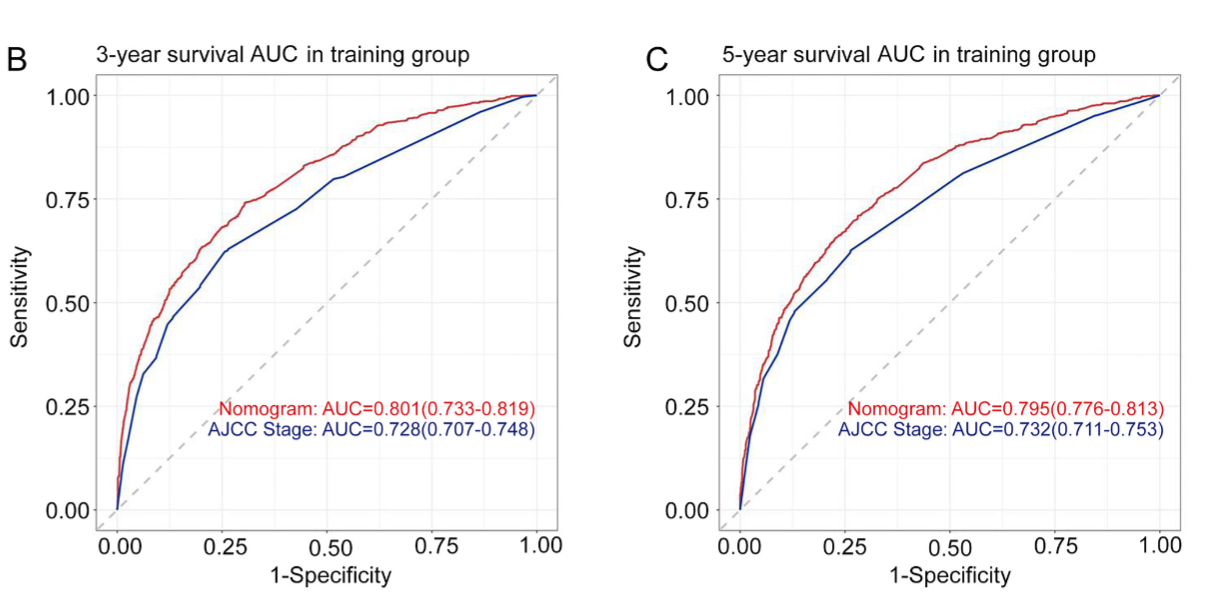
A nomogram was constructed to predict the survival of patients with colorectal mucinous adenocarcinoma based on data extracted from the Surveillance, Epidemiology and End Result (SEER) database. Data collected between 2010 and 2018 were obtained from the SEER database. The log-rank test and multivariate Cox regression were performed to identify the independent prognostic factors for overall survival, which were further used to construct a nomogram model to predict 1-, 3-, and 5-year overall survival. In total, 10846 patients diagnosed with colorectal mucinous adenocarcinoma were enrolled in the study. The following 11 variables were associated with survival and were further incorporated into the nomogram: age at diagnosis, primary site, grade, tumour size, lymph node dissection, T stage, N stage, M stage, surgery for primary site, chemotherapy, and household income. The concordance index (C-index) value was 0.725 (95% confidence interval 0.716-0.734), and the receiver operating characteristic curves and calibration curves showed satisfactory predictive accuracy. Both the C-index and time-independent area under the curve values were greater than those of the American Joint Committee on Cancer 7th TNM classification system (both P < 0.001). In the validation group, the results were consistent with those of the training group, with a C-index value of 0.726 (95% confidence interval 0.713-0.739). This study constructed a practical nomogram to predict 1-, 3-, and 5-year OS for patients with colorectal colorectal mucinous adenocarcinoma based on SEER data.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Qian Wu, Suqin Zhang, Huan Wang, Yifei Zeng, Wei Yang, Wenjun Pan, Guodai Hong, Wenbin Gao

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-11-08
Published 2023-05-01









