Time to diagnostic certainty for saddle pulmonary embolism in hospitalized patients
DOI:
https://doi.org/10.17305/bb.2022.8393Keywords:
Pulmonary embolism, performance measure, time to diagnosis, diagnostic error, diagnostic delay, electronic health recordsAbstract
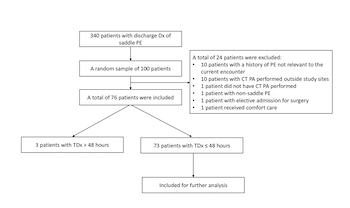
There is a lack of diagnostic performance measures associated with pulmonary embolism (PE). We aimed to explore the concept of the time to diagnostic certainty, which we defined as the time interval that elapses between first presentation of a patient to a confirmed PE diagnosis with computed tomography pulmonary angiogram (CT PA). This approach could be used to highlight variability in health system diagnostic performance, and to select patient outliers for structured chart review in order to identify underlying contributors to diagnostic error or delay. We performed a retrospective observational study at academic medical centers and associated community-based hospitals in one health system, examining randomly selected adult patients admitted to study sites with a diagnosis of acute saddle PE. One hundred patients were randomly selected from 340 patients discharged with saddle PE. Twenty-four patients were excluded. Among the 76 included patients, time to diagnostic certainty ranged from 1.5 to 310 hours. We found that 73/76 patients were considered to have PE present on admission (CT PA ≤ 48 hours). The proportion of patients with PE present on admission with time to diagnostic certainty of > 6 hours was 26% (19/73). The median (IQR) time to treatment (thrombolytics/anticoagulants) was 3.5 (2.5-5.1) hours among the 73 patients. The proportion of patients with PE present on admission with treatment delays of > 6 hours was 16% (12/73). Three patients acquired PE during hospitalization (CT PA > 48 hours). In this study, we developed and successfully tested the concept of time to diagnostic certainty for saddle PE.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Yuliya Pinevich, Amelia K. Barwise, John Matthew Austin, Jalal Soleimani, Svetlana Herasevich, Sarah Redmond , Yue Dong , Vitaly Herasevich , Ognjen Gajic, Brian W. Pickering

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-01-10
Published 2023-07-03









