Is hepatic resection always a better choice than radiofrequency ablation for solitary hepatocellular carcinoma regardless of age and tumor size?
DOI:
https://doi.org/10.17305/bb.2022.8507Keywords:
Hepatocellular carcinoma, hepatic resection, radiofrequency ablation, tumor size, elderly, SEERAbstract
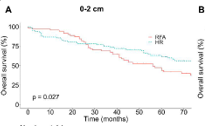
In this study, we aimed to compare survival outcomes after receiving radiofrequency ablation (RFA) and hepatic resection (HR) for solitary hepatocellular carcinoma (HCC) with stratification by tumor size and age. A retrospective cohort was obtained from the Surveillance, Epidemiology, and End Results (SEER) database from 2004 to 2015. Patients were grouped by tumor size (0-2, 2-5, and > 5 cm) and age (>65 and ≤65). Overall survival (OS) and disease-specific survival (DSS) were assessed. For patients >65 with tumors measuring 0-2 and 2-5 cm, the HR group had better OS and DSS compared with the RFA group. For patients >65 with tumors > 5 cm, OS and DSS did not differ significantly between the RFA and HR groups (p = 0.262 and p = 0.129, respectively). For patients ≤65, the HR group had better OS and DSS compared with the RFA group regardless of tumor size. For patients with resectable solitary HCC, regardless of age, HR is the better choice not only for tumors ≤ 2 cm, but also for tumors 2-5 cm. For resectable solitary HCC with tumors >5 cm, HR is the better choice for patients ≤65 but for patients >65, the issue of treatment choice needs to be further studied.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Shicong Zeng, Yao Zhang, Zongwen Wang, Xiaohang Ren, Jingtao Li, Shuoheng Ma, Wenyu Liu, Qiankun Zhu, Yan Yan, Bo Zhai

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-02-21
Published 2023-07-03









