The relationship between labial soft tissue changes and jumping spaces after immediate implant placement and restoration in the anterior maxilla: A prospective study
DOI:
https://doi.org/10.17305/bb.2023.8652Keywords:
Immediate implant insertion/placement, jumping space , jumping gap, immediate provisionalization, soft tissue, esthetic regionAbstract
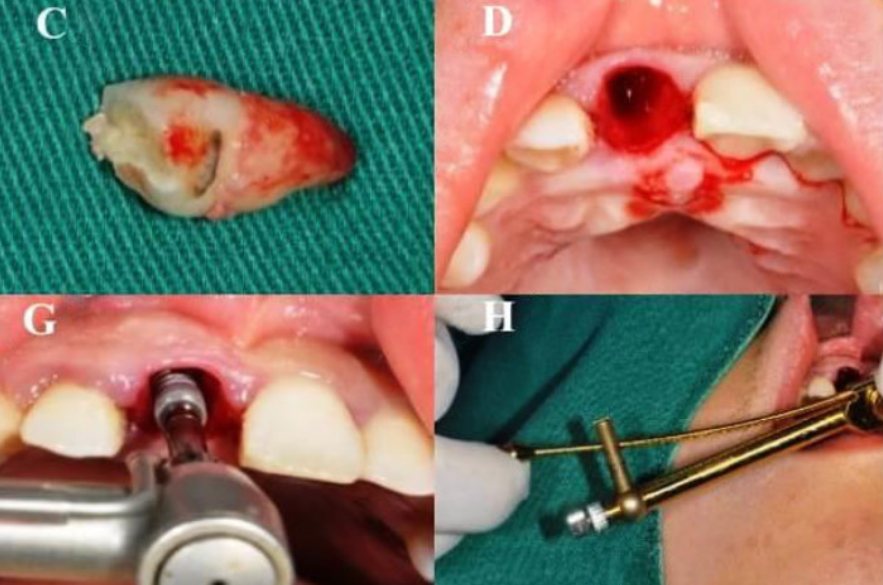
Oral implants have been increasingly used in the treatment of edentulous patients or those with dentition defects due to reliable treatment procedure and favorable long-term prognosis. We investigated the changes of labial soft tissue contours with different jumping spaces after immediate implant placement and restoration (IIPR) in the maxillary esthetic area and also provided a long-term stability measurement for the changing trend of soft tissue contour. All patients had been separated into three groups based on the jumping space: group A (horizontal defect dimension [HDD] 2 mm), group B (2 mm < HDD 3 mm), and group C (HDD > 3 mm) and the digital impressions were obtained in the first, third, and sixth month after the operation. The changes of gingival mucosa levels, the average thickness of soft tissue contour volume, and the linear change of submarginal level decreased gradually across the three groups, with the largest change of submarginal level being at 5mm. The size of the jumping space was moderately negatively correlated with the level and average thickness of gingival mucosa and the linear changes of 3 mm and 5 mm under gingival margin, while there was no significant correlation with pink esthetic score (PES) and the linear change of the 1 mm under the gingival margin. Generally, IIPR of upper anterior teeth can achieve esthetic satisfaction, and the level of soft tissue around the implant can be well preserved.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Linkun Xu, Sui Zhang, Yue Chen, Feiyan Yu, Chong Han, Dongchao Wu, Dongning He

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-03-20
Published 2023-09-04









