Myocarditis and coronavirus disease 2019 vaccination: A systematic review and meta-summary of cases
DOI:
https://doi.org/10.17305/bb.2022.8779Keywords:
myocarditis, COVID-19 vaccination, vaccine, SARS-CoV2, side effectAbstract
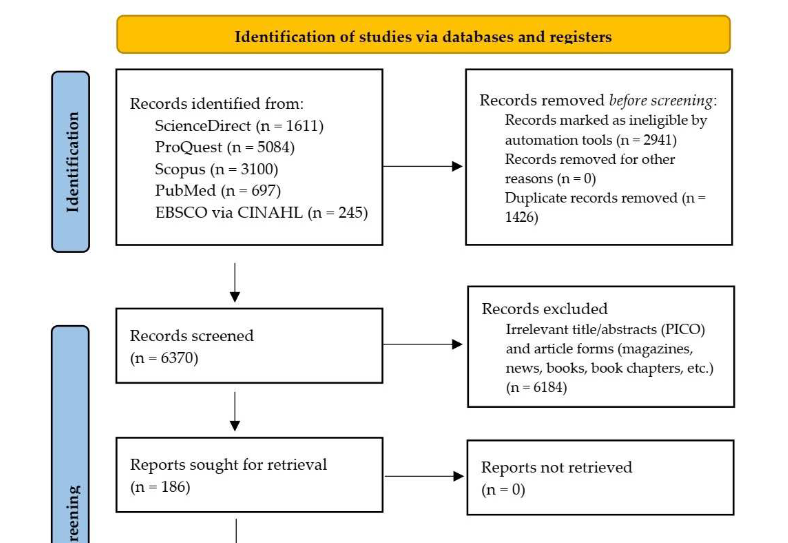
Vaccination is significant to control, mitigate, and recover from the destructive effects of coronavirus disease 2019 (COVID-19). The incidence of myocarditis following COVID-19 vaccination has been increasing and growing public concern; however, little is known about it. This study aimed to systematically review myocarditis following COVID-19 vaccination. We included studies containing individual patient data of myocarditis following COVID-19 vaccination published between January 1, 2020 and September 7, 2022 and excluded review articles. Joanna Briggs Institute critical appraisals were used for risk of bias assessment. Descriptive and analytic statistics were performed. A total of 121 reports and 43 case series from five databases were included. We identified 396 published cases of myocarditis and observed that the majority of cases was male patients, happened following the second dose of mRNA vaccine administration, and experienced chest pain as a symptom. Previous COVID-19 infection was significantly associated (p < 0.01; OR, 5.74; 95% CI, 2.42–13.64) with the risk of myocarditis following the administration of the first dose, indicating that its primary mechanism is immune-mediated. Moreover, 63 histopathology examinations were dominated by non-infective subtypes. Electrocardiography and cardiac marker combination is a sensitive screening modality. However, cardiac magnetic resonance is a significant noninvasive examination to confirm myocarditis. Endomyocardial biopsy may be considered in confusing and severe cases. Myocarditis following COVID-19 vaccination is relatively benign, with a median length of hospitalization of 5 days, intensive care unit admission of <12%, and mortality of <2%. The majority was treated with nonsteroidal anti-inflammatory drugs, colchicine, and steroids. Surprisingly, deceased cases had characteristics of being female, older age, non-chest pain symptoms, first-dose vaccination, left ventricular ejection fraction of <30%, fulminant myocarditis, and eosinophil infiltrate histopathology.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Pandit Bagus Tri Saputra, Roy Bagus Kurniawan, Desy Trilistyoati, Makhyan Jibril Al Farabi, Hendri Susilo, Mochamad Yusuf Alsagaff, Yudi Her Oktaviono, Henry Sutanto, Arief Gusnanto, Citrawati Dyah Kencono Wungu

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-02-16
Published 2023-07-03









