Serum irisin correlates to the severity of acute myocardial infarction and predicts the postoperative major adverse cardiovascular events
DOI:
https://doi.org/10.17305/bb.2023.8888Keywords:
Acute myocardial infarction (AMI), percutaneous coronary intervention (PCI), major adverse cardiovascular events (MACE), irisin, ROC curveAbstract
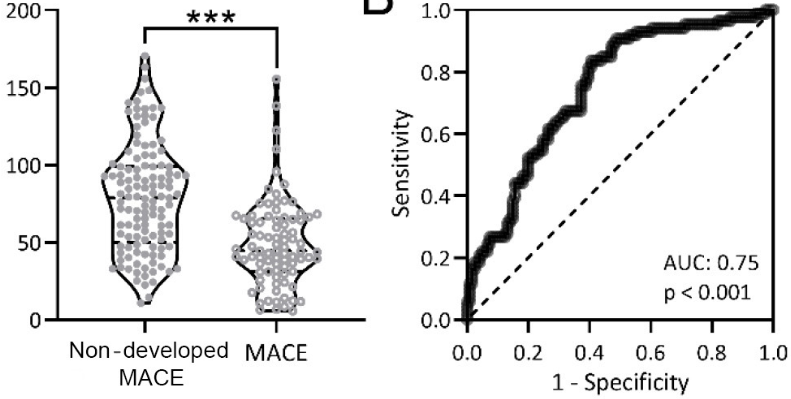
Irisin is a myogenic cytokine which plays an important role in the cardiovascular system. The aim of this study was to investigate the correlation between serum irisin levels and major adverse cardiovascular events (MACE) in patients with acute myocardial infarction (AMI) after percutaneous coronary intervention (PCI). A total of 207 patients with AMI who underwent PCI were selected as research subjects. Serum irisin levels at admission were measured, and patients were stratified according to the receiver operating characteristic curve to assess differences in MACE within one year after PCI. After one year of follow-up, 207 patients were divided into two groups, 86 with MACE and 121 without MACE. There were significant differences in age, Killip grade, left ventricular ejection fraction, cardiac troponin I, creatine kinase-muscle/brain, and serum irisin between the two groups. Serum irisin level at admission in AMI patients significantly correlated with the occurrence of MACE after PCI, and could be used as an effective marker for predicting the occurrence of MACE in AMI patients after PCI.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Qiaoying Chai , Wei Zhang , Lijuan Gao, Yingtao Yang, Shuanli Xin

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-04-11
Published 2023-09-04









