Small intestinal submucosa promotes angiogenesis via the Hippo pathway to improve vaginal repair
DOI:
https://doi.org/10.17305/bb.2023.9052Keywords:
Vaginal reconstruction, small intestinal submucosa (SIS), Hippo pathway, large animalsAbstract
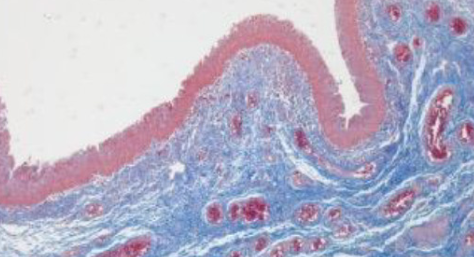
Vaginal reconstruction has incorporated the use of gastrointestinal segments for decades, but the technique is inevitably associated with complications. Tissue-engineering techniques, however, have brought great hope for vaginal reconstruction. This study aimed to evaluate the utility of small intestinal submucosa (SIS) in reconstructing clinically significant large vaginal defects in a porcine model and to investigate the role of the Hippo pathway in the vascular remodeling process. The composition and mechanical properties of SIS were characterized. Full-thickness vaginal defects were established in 10 minipig donors, with 4 cm lengths removed and replaced by an equal sized SIS scaffolds. The neovaginas were subjected to macroscopic, histological, immunohistochemical and molecular evaluations at 4 and 12 weeks after the surgery. Four weeks after the operation, extracellular matrix reorganization and complete coverage of the surface of the luminal matrix by vaginal epithelium were observed, accompanied by the formation of a microvascular network and the regeneration of smooth muscles, albeit disorderly arranged. Twelve weeks after implantation, enhancements were seen in the formation of the multi-layered squamous epithelium, angiogenesis, and large muscle bundle formation in the vagina. Additionally, the expression levels of angiogenesis-related proteins, proliferation-related proteins and Hippo pathway-related proteins in the neovagina were significantly increased. These results indicate that SIS could be used to reconstruct large vaginal defects and that the vascular remodeling process is potentially regulated by the Hippo pathway.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Yanlai Xiao, Yanpeng Tian, Jingkun Zhang, Qian Li, Wenxin Shi , Xianghua Huang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-04-19
Published 2023-09-04









