The mouse prostate: a basic anatomical and histological guideline
DOI:
https://doi.org/10.17305/bjbms.2016.917Keywords:
mouse models, prostate, prostate cancer, mouse prostate histology, mouse prostate anatomyAbstract
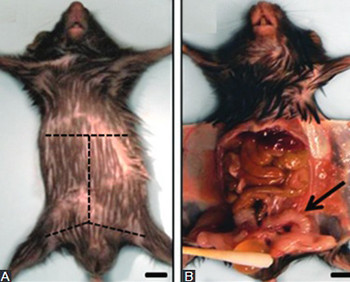
Despite substantial similarities in embryological, cellular and molecular biology features, human and mouse prostates differ in organ morphology and tissue architecture. Thus, a clear understanding of the anatomy and histology of the mouse prostate is essential for the identification of urogenital phenotypes in genetically engineered mice, as well as for the study of the etiology, development, and treatment of human prostatic diseases for which mouse models are used. The purpose of this manuscript is to provide a brief guide for the dissection of the mouse prostate and the identification of its different lobes and histology, to both basic researchers and medical pathologists who are unfamiliar with mouse tissues.
Citations
Downloads
References
Roehrborn C. Insights into the relationships between prostatic disorders and their potential impact on future urologic practice. Eur Urol. 2013;Suppl. 5:698-703.
Luke MC, Coffey DS. The male sex accessory tissues. In: Knobil E, Nelly JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. p. 1435-89.
Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165-74. http://dx.doi.org/10.1016/S0012-1606(02)00031-3
Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151(5):1427-32.
Prins GS, Lindgren M. Accessory sex glands in the male. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's physiology of reproduction. 1. 4th ed. London, UK: Elsevier; 2015. p. 773-804. http://dx.doi.org/10.1016/b978-0-12-397175-3.00018-1
Leroy BE, Northrup N. Prostate cancer in dogs: comparative and clinical aspects. Vet J. 2009;180(2):149-62.
http://dx.doi.org/10.1016/j.tvjl.2008.07.012
Rosol TJ, Tannehill-Gregg SH, LeRoy BE, Mandl S, Contag CH. Animal models of bone metastasis. Cancer. 2003;97(3 Suppl):748-57.
http://dx.doi.org/10.1002/cncr.11150
Danneman PJ, Brayton CF, Suckow MA. The Laboratory Mouse. Suckow MA, editor. United States of America: CRC Press LLC; 2000. 168 p.
Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat Cell Biol. 2007;9(9):993-9.
http://dx.doi.org/10.1038/ncb437
Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011;6:95-119.
http://dx.doi.org/10.1146/annurev.pathol.3.121806.154244
de Jong M, Maina T. Of mice and humans: are they the same?--Implications in cancer translational research. J Nucl Med. 2010;51(4):501-4.
http://dx.doi.org/10.2967/jnumed.109.065706
Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447(7147):966-71.
http://dx.doi.org/10.1038/nature05886
Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8(3):338-62.
http://dx.doi.org/10.1210/edrv-8-3-338
Donjacour AA, Cunha GR. The effect of androgen deprivation on branching morphogenesis in the mouse prostate. Dev Biol. 1988;128(1):1-14.
http://dx.doi.org/10.1016/0012-1606(88)90260-6
Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34(5):961-71.
http://dx.doi.org/10.1095/biolreprod34.5.961
Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, et al. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232(2):301-14.
http://dx.doi.org/10.1006/dbio.2001.0187
Pu Y, Huang L, Birch L, Prins GS. Androgen regulation of prostate morphoregulatory gene expression: Fgf10-dependent and -independent pathways. Endocrinology. 2007;148(4):1697-706.
http://dx.doi.org/10.1210/en.2006-1113
Zhou X. Roles of androgen receptor in male and female reproduction: lessons from global and cell-specific androgen receptor knockout (ARKO) mice. J Androl. 2010;31(3):235-43.
http://dx.doi.org/10.2164/jandrol.109.009266
Chang C, Lee SO, Wang RS, Yeh S, Chang TM. Androgen receptor (AR) physiological roles in male and female reproductive systems: lessons learned from AR-knockout mice lacking AR in selective cells. Biol Reprod. 2013;89(1):21. http://dx.doi.org/10.1095/biolreprod.113.109132
Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007;10(1):15-29.
http://dx.doi.org/10.1038/sj.pcan.4500930
Schwartz ES, Xie A, La JH, Gebhart GF. Nociceptive and inflammatory mediator upregulation in a mouse model of chronic prostatitis. Pain. 2015;156(8):1537-44. http://dx.doi.org/10.1097/j.pain.0000000000000201
Irshad S, Abate-Shen C. Modeling prostate cancer in mice: something old, something new, something premalignant, something metastatic. Cancer Metastasis Rev. 2013;32(1-2):109-22. http://dx.doi.org/10.1007/s10555-012-9409-1
Grabowska MM, DeGraff DJ, Yu X, Jin RJ, Chen Z, Borowsky AD, et al. Mouse models of prostate cancer: picking the best model for the question. Cancer Metastasis Rev. 2014;33(2-3):377-97. http://dx.doi.org/10.1007/s10555-013-9487-8
Wu X, Gong S, Roy-Burman P, Lee P, Culig Z. Current mouse and cell models in prostate cancer research. Endocr Relat Cancer. 2013;20(4):R155-70.
http://dx.doi.org/10.1530/ERC-12-0285
Kuroda N, Naroda T, Tamura M, Perez-Montiel D, Michal M, Hes O. High-grade urothelial carcinoma, plasmacytoid variant, of the renal pelvis with osteoclast-like giant cells and focal rhabdoid features. Polish journal of pathology : official journal of the Polish Society of Pathologists. 2014;65(3):237-40. http://dx.doi.org/10.5114/pjp.2014.45788
McNeal JE. Regional morphology and pathology of the prostate. Am J Clin Pathol. 1968;49(3):347-57.
McNeal JE. Prostate. In: Sternberg SS, editor. Histology for pathologists. 2nd ed. Philadelphia: Lipincott; 1997. p. 997-1017.
Price D. Comparative Aspects of Development and Structure in the Prostate. Natl Cancer Inst Monogr. 1963;12:1-27.
Berquin IM, Min Y, Wu R, Wu H, Chen YQ. Expression signature of the mouse prostate. J Biol Chem. 2005;280(43):36442-51. http://dx.doi.org/10.1074/jbc.M504945200
McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897-906. http://dx.doi.org/10.1097/00000478-198812000-00001
Reissigl A, Pointner J, Strasser H, Ennemoser O, Klocker H, Bartsch G. Frequency and clinical significance of transition zone cancer in prostate cancer screening. Prostate. 1997;30(2):130-5.
http://dx.doi.org/10.1002/(SICI)1097-0045(19970201)30:2<130::AID-PROS8>3.0.CO;2-S
Noguchi M, Stamey TA, Neal JE, Yemoto CE. An analysis of 148 consecutive transition zone cancers: clinical and histological characteristics. J Urol. 2000;163(6):1751-5.
http://dx.doi.org/10.1016/S0022-5347(05)67535-0
De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256-69. http://dx.doi.org/10.1038/nrc2090
Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocrine-Related Cancer. 2004;11(2):225-54. http://dx.doi.org/10.1677/erc.0.0110225
Bostwick DG, Cheng L. Urological Surgical Pathology. 3rd ed. Philadelphia: Elsevier Health Sciences; 2014.
Zhang C, Guo Y, Cui J, Zhu HH, Gao WQ. Cytokeratin 18 is not required for morphogenesis of developing prostates but contributes to adult prostate regeneration. Biomed Res Int. 2013;2013:576472. http://dx.doi.org/10.1155/2013/576472
Lee SO, Tian J, Huang CK, Ma Z, Lai KP, Hsiao H, et al. Suppressor role of androgen receptor in proliferation of prostate basal epithelial and progenitor cells. J Endocrinol. 2012;213(2):173-82.
http://dx.doi.org/10.1530/JOE-11-0474
Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104(1):181-6.
http://dx.doi.org/10.1073/pnas.0609684104
Diamandis EP, Yousef GM, Olsson AY. An update on human and mouse glandular kallikreins. Clin Biochem. 2004;37(4):258-60. http://dx.doi.org/10.1016/j.clinbiochem.2003.12.013
Fujimoto N, Akimoto Y, Suzuki T, Kitamura S, Ohta S. Identification of prostatic-secreted proteins in mice by mass spectrometric analysis and evaluation of lobe-specific and androgen-dependent mRNA expression. J Endocrinol. 2006;190(3):793-803. http://dx.doi.org/10.1677/joe.1.06733
Zhou Z, Flesken-Nikitin A, Nikitin AY. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res. 2007;67(12):5683-90. http://dx.doi.org/10.1158/0008-5472.CAN-07-0768
Knoblaugh S, True L. Male reproductive system. In: Treuting PM, Dintzis SM, editors. Comparative anatomy and histology A mouse and human atlas. 1st ed. London, UK: Academic Press; 2012. p. 285-308. http://dx.doi.org/10.1016/B978-0-12-381361-9.00018-4
Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64(6):2270-305.
http://dx.doi.org/10.1158/0008-5472.CAN-03-0946
Harmelin A, Danon T, Kela I, Brenner O. Biopsy of the mouse prostate. Lab Anim. 2005;39(2):215-20.
http://dx.doi.org/10.1258/0023677053739756
Scudamore CL. Reproductive system. In: Scudamore CL, editor. A practical guide to the histology of the mouse. Hoboken, NJ: Wiley Blackwell; 2014. p. 87-107.
http://dx.doi.org/10.1002/9781118789568
Suwa T, Nyska A, Haseman JK, Mahler JF, Maronpot RR. Spontaneous lesions in control B6C3F1 mice and recommended sectioning of male accessory sex organs. Toxicol Pathol. 2002;30(2):228-34.
Downloads
Additional Files
Published
How to Cite
Accepted 2015-12-04
Published 2016-02-10









