Prognostic value of a decrease in mean platelet volume, platelet distribution width, and platelet-large cell ratio for major adverse cardiovascular events after myocardial infarction without ST-segment elevation: An observational study
DOI:
https://doi.org/10.17305/bb.2023.9178Keywords:
Mean platelet volume (MPV), platelet distribution width (PDW), platelet-large cell ratio (P-LCR), major adverse cardiovascular events (MACE), myocardial infarction without ST-segment elevationAbstract
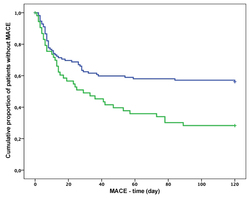
The current study aimed to explore whether the level of decrease in platelet distribution width (PDW), platelet-large cell ratio (P-LCR), and mean platelet volume (MPV) has prognostic value for major adverse cardiovascular events (MACEs) in acute myocardial infarction without ST-segment elevation (NSTEMI) treated with clopidogrel. In this prospective observational cohort study, PDW, P-LCR, and MPV were determined on admission at the hospital and 24 h after clopidogrel treatment in 170 non-STEMI patients. MACEs were assessed over a one-year follow-up period. Using the Cox regression test, a decrease in PDW showed a significant association with the incidence of MACEs (odds ratio [OR] 0.82, 95% confidence interval [CI] 0.66–0.99, p = 0.049) and overall survival rate (OR 0.95, 95% CI = 0.91–0.99, p = 0.016). Patients with a decrease in PDW<9.9% had a higher incidence of MACEs (OR 0.42, 95% CI = 0.24–0.72, p = 0.002) and a lower survival rate (OR 0.32, 95% CI = 0.12–0.90, p = 0.03) than patients who had a decrease in PDW < 9.9%. In the Kaplan–Meier analysis using log-rank test, patients who had a decrease in PDW < 9.9% had an increased risk for MACEs (p = 0.002) and lethal outcomes (p = 0.002). However, a decrease in MPV or P-LCR did not have prognostic value. A decrease in PDW < 9.9% measured 24 h after clopidogrel treatment in NSTEMI patients has good prognostic value for determining the short-term risks of MACEs, possibly providing a better risk stratification of those patients.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2023 Emir Bećirović, Kenana Ljuca, Minela Bećirović, Nadina Ljuca, Mugdim Bajrić, Ammar Brkić, Farid Ljuca

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-05-14
Published 2023-09-04









