Lithium chloride could aggravate brain injury caused by 3-nitropropionic acid
DOI:
https://doi.org/10.17305/bjbms.2016.1206Keywords:
Lithium chloride, 3-nitropropionic acid, cytochrome c oxidase, synaptotagmin-4, synaptotagmin-7Abstract
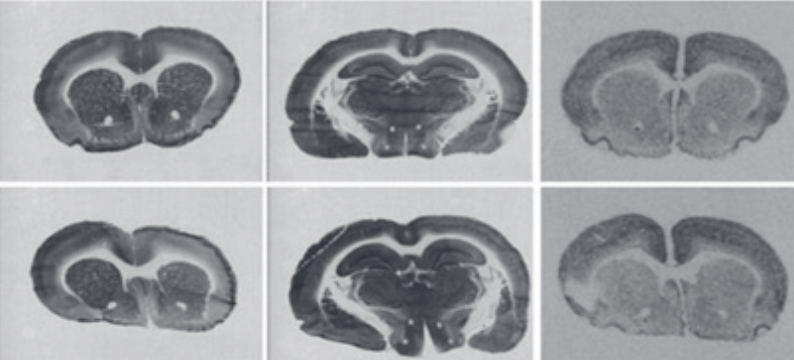
Lithium, a well-known drug for the treatment of bipolar disorder, may also have the ability to reduce neurodegeneration and stimulate cell proliferation. Systemic injection of mitochondrial toxin 3-nitropropionic acid (3NPA) is known to induce a relatively selective, Huntington disease-like brain injury. The aim of this study was to determine the effect of lithium chloride (LiCl) on brain injury caused by 3NPA. Female adult Wistar rats were pre-treated with LiCl (127 mg/kg) 1 day before the first injection of 3NPA (28 mg/kg), and then for 8 days with the same treatment but receiving LiCl 1 hour before 3NPA. Control groups were pre-treated accordingly, with LiCl or with normal saline, but were not treated with 3NPA. Staining for cytochrome c oxidase activity and in situ hybridization autoradiography of synaptotagmin-4 and -7 mRNAs were used to evaluate brain injury caused by 3NPA. There was a significant reduction of body weight in the 3NPA+LiCl group (79%) compared to the 3NPA group (90%, p = 0.031) and both control groups (100%, p = 0.000). Densitometric evaluation of cytochrome c oxidase staining and in situ hybridization autoradiograms revealed that the pre-treatment with LiCl caused an increase in striatal lesion for about 40% (p = 0.049). Moreover, the lesion was observed also in the hippocampus of three animals from the 3NPA+LiCl group and in two animals from the 3NPA group. However, there were no differences between the LiCl and saline group in any of the measured parameters. We concluded that the pre-treatment with a relatively nontoxic dose of LiCl could aggravate brain injury caused by 3NPA.
Citations
Downloads
References
. Diniz BS, Machado-Vieira R, Forlenza OV. Lithium and neuroprotection: Translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. http://dx.doi.org/10.2147/NDT.S33086.
. Machado-Vieira R, Manji HK, Zarate CA Jr. The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11 Suppl 2:92-109.
http://dx.doi.org/10.1111/j.1399-5618.2009.00714.x.
. Forlenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF. Does lithium prevent Alzheimer's disease? Drugs Aging. 2012;29(5):335-42.
http://dx.doi.org/10.2165/11599180-000000000-00000.
. Forlenza OV, De-Paula VJ, Diniz BS. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer's disease and related neurodegenerative disorders. ACS Chem Neurosci. 2014;5(6):443-50. http://dx.doi.org/10.1021/cn5000309.
. Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington's disease. Mol Psychiatry. 2004;9(4):371-85. http://dx.doi.org/10.1038/sj.mp.4001463.
. Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95(5):2642-7. http://dx.doi.org/10.1073/pnas.95.5.2642.
. James LF, Hartley WJ, Williams MC, Van Kampen KR. Field and experimental studies in cattle and sheep poisoned by nitro-bearing Astragalus or their toxins. Am J Vet Res. 1980;41(3):377-82.
. Liu X, Luo X, Hu W. Studies on the epidemiology and etiology of moldy sugarcane poisoning in China. Biomed Environ Sci. 1992;5(2):161-77.
. Ming L. Moldy sugarcane poisoning – A case report with a brief review. J Toxicol Clin Toxicol. 1995;33(4):363-7. http://dx.doi.org/10.3109/15563659509028924.
. Ramaswamy S, McBride JL, Kordower JH. Animal models of Huntington's disease. ILAR J. 2007;48(4):356-73. http://dx.doi.org/10.1093/ilar.48.4.356.
. Mirandola SR, Melo DR, Saito A, Castilho RF. 3-nitropropionic acid-induced mitochondrial permeability transition: Comparative study of mitochondria from different tissues and brain regions. J Neurosci Res. 2010;88(3):630-9.
. McCracken E, Dewar D, Hunter AJ. White matter damage following systemic injection of the mitochondrial inhibitor 3-nitropropionic acid in rat. Brain Res. 2001;892(2):329-35. http://dx.doi.org/10.1016/S0006-8993(00)03266-2.
. Szabó A, Papp A, Nagymajtényi L. Effects of 3-nitropropionic acid in rats: General toxicity and functional neurotoxicity. Arh Hig Rada Toksikol. 2005;56(4):297-302.
. Gabrielson KL, Hogue BA, Bohr VA, Cardounel AJ, Nakajima W, Kofler J, et al. Mitochondrial toxin 3-nitropropionic acid induces cardiac and neurotoxicity differentially in mice. Am J Pathol. 2001;159(4):1507-20.
http://dx.doi.org/10.1016/S0002-9440(10)62536-9.
. Milutinovic A, Zorc-Pleskovic R. Glycogen accumulation in cardiomyocytes and cardiotoxic effects after 3NPA treatment. Bosn J Basic Med Sci. 2012;12(1):15-9.
. Glavan G, See RE, Živin M. Differential patterns of synaptotagmin7 mRNA expression in rats with kainate- and pilocarpine-induced seizures. PLoS One. 2012;7(5):e36114. http://dx.doi.org/10.1097/WNR.0b013e3280ef6964.
. Saghafi MM, Pregelj P, Zivin M. Donepezil inhibits diisopropylfluorophosphate-induced seizures and up-regulation of synaptotagmin 4 mRNA. Folia Biol (Praha). 2010;56(6):256-62.
. Glisovic S, Glavan G, Saghafi MM, Zivin M. Upregulation of synaptotagmin IV protein in kainate-induced seizures. Neuroreport. 2007;18(8):831-5.
http://dx.doi.org/10.1097/WNR.0b013e3280ef6964.
. Glavan G, Zivin M. Differential expression of striatal synaptotagmin mRNA isoforms in hemiparkinsonian rats. Neuroscience 2005;135(2):545-54.
http://dx.doi.org/10.1016/j.neuroscience.2005.05.050.
. Glavan G, Schliebs R, Zivin M. Synaptotagmins in neurodegeneration. Anat Rec (Hoboken). 2009;292(12):1849-62. http://dx.doi.org/10.1002/ar.21026.
. Tratnjek L, Zivin M, Glavan G. Up-regulation of Synaptotagmin IV within amyloid plaque-associated dystrophic neurons in Tg2576 mouse model of Alzheimer's disease. Croat Med J. 2013;54(5):419-28.
http://dx.doi.org/10.3325/cmj.2013.54.419.
. Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171(1):11-28. http://dx.doi.org/10.1016/0006-8993(79)90728-5.
. Zivin M, Milatovic D, Dettbarn WD. Nitrone spin trapping compound N-tert-butyl-alpha-phenylnitrone prevents seizures induced by anticholinesterases. Brain Res. 1999;850(1-2):63-72. http://dx.doi.org/10.1016/S0006-8993(99)02101-0.
. Crespo-Biel N, Camins A, Pallàs M, Canudas AM. Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro. Neuropharmacology. 2009;56(2):422-8.
http://dx.doi.org/10.1016/j.neuropharm.2008.09.012.
. Khan A, Jamwal S, Bijjem KR, Prakash A, Kumar P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3ß modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience. 2015;287(3):66-77. http://dx.doi.org/10.1016/j.neuroscience.2014.12.018.
. Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience. 1987;23:953-68. http://dx.doi.org/10.1016/0306-4522(87)90171-0.
. Reddy DS, Kuruba R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 2013;14(9):18284-318. http://dx.doi.org/10.3390/ijms140918284.
. Rang HP, Dale MM, Ritter JM, Flower RJ. Antidepressant drugs. Rang and Dales Pharmacology. 6th ed. London, England: Elsevier Churchill Livingstone; 2008. p. 569-73.
. Serdarevic N, Kozjek F, Malesic I. Saliva and serum lithium monitoring in hospitalized patients and possibility to replace serum to saliva. Bosn J Basic Med Sci. 2006;6(4):32-5.
. Serdarevic N, Malesic I, Kozjek F. Comparison of vitros dry slide technology for determination of lithium ions with other methods. Bosn J Basic Med Sci. 2006;6(2):32-6.
. Yamantürk-Çelik P, Unlüçerçi Y, Sevgi S, Bekpinar S, Eroglu L. Nitrergic, glutamatergic and gabaergic systems in lithium toxicity. J Toxicol Sci. 2012;37(5):1017-23.
http://dx.doi.org/10.2131/jts.37.1017.
. Dimitrova M, Petrova E, Gluhcheva Y, Kadiysky D, Dimitrova S, Kolyovska V, et al. Neurodegenerative changes in rat produced by lithium treatment. J Toxicol Environ Health A. 2013;76(4-5):304-10.
http://dx.doi.org/10.1080/15287394.2013.757268.
. Hirvonen MR. Cerebral lithium, inositol and inositol monophosphates. Pharmacol Toxicol. 1991;69(1):22-7. http://dx.doi.org/10.1111/j.1600-0773.1991.tb00403.x.
Downloads
Additional Files
Published
License
Copyright (c) 2016 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2016-04-23
Published 2016-11-10









