A remarkable year for NSCLC: Seven new FDA approvals in 2025 across molecular targets
DOI:
https://doi.org/10.17305/bb.2026.13832Keywords:
NSCLC, precision oncology, biomarker-driven therapy, ADC, TKIAbstract
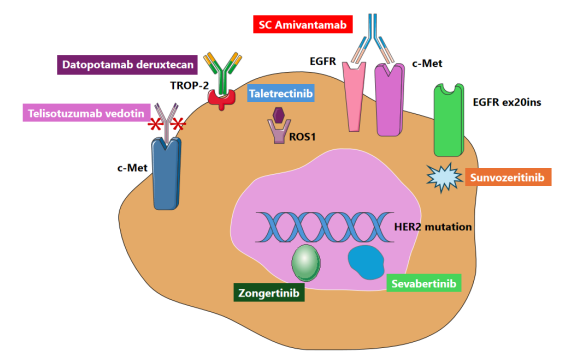
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer mortality worldwide; however, precision oncology has fundamentally transformed its treatment landscape. In 2025, seven approvals by the U.S. Food and Drug Administration (FDA) further accelerated biomarker-driven care across critical molecular subsets. These include MET-directed and trophoblast cell-surface antigen-2 (TROP-2) antibody-drug conjugates (ADCs), expanded strategies targeting epidermal growth factor receptor (EGFR), notably those addressing exon 20 insertion mutations, a ROS proto-oncogene 1 (ROS1) inhibitor, and various human epidermal growth factor receptor 2 (HER2) options that encompass both tumor-agnostic and mutation-selected approaches. These advancements underscore the necessity for integrated diagnostics—such as next-generation sequencing (NGS), fluorescence in situ hybridization (FISH), and immunohistochemistry (IHC)—while also emphasizing ongoing challenges in biomarker selection, therapeutic sequencing, and equitable global implementation.
Citations
Downloads
References
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45.
https://doi.org/10.1186/s12943-018-0796-y
U.S. Food and Drug Administration. FDA grants accelerated approval to telisotuzumab vedotin-tllv for NSCLC with high c-MET protein overexpression. Silver Spring (MD): FDA; 2025 May 8 [cited 2025 Dec 29].
Camidge DR, Bar J, Horinouchi H, Goldman J, Moiseenko F, Filippova E, et al. Telisotuzumab vedotin monotherapy in patients with previously treated c-Met protein-overexpressing advanced nonsquamous EGFR-wildtype non-small cell lung cancer in the phase II LUMINOSITY trial. J Clin Oncol. 2024;42(25):3000–11.
https://doi.org/10.1200/JCO.24.00720
Vranic S, Gatalica Z. Trop-2 protein as a therapeutic target: a focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers. Bosn J Basic Med Sci. 2022;22(1):14–21.
https://doi.org/10.17305/bjbms.2021.6100
U.S. Food and Drug Administration. FDA grants accelerated approval to datopotamab deruxtecan-dlnk for EGFR-mutated non-small cell lung cancer. Silver Spring (MD): FDA; 2025 [cited 2025 Dec 29].
Sands J, Ahn MJ, Lisberg A, Cho BC, Blumenschein G, Shum E, et al. Datopotamab deruxtecan in advanced or metastatic non-small cell lung cancer with actionable genomic alterations: results from the phase II TROPION-Lung05 study. J Clin Oncol. 2025;43(10):1254–65.
https://doi.org/10.1200/JCO-24-01349
Ahn MJ, Tanaka K, Paz-Ares L, Cornelissen R, Girard N, Pons-Tostivint E, et al. Datopotamab deruxtecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: the randomized, open-label phase III TROPION-Lung01 study. J Clin Oncol. 2025;43(3):260–72.
U.S. Food and Drug Administration. FDA approves amivantamab and hyaluronidase-lpuj subcutaneous injection. Silver Spring (MD): FDA; 2025 Dec 17 [cited 2025 Dec 29].
Leighl NB, Akamatsu H, Lim SM, Cheng Y, Minchom AR, Marmarelis ME, et al. Subcutaneous versus intravenous amivantamab, both in combination with lazertinib, in refractory epidermal growth factor receptor-mutated non-small cell lung cancer: primary results from the phase III PALOMA-3 study. J Clin Oncol. 2024;42(30):3593–605.
https://doi.org/10.1200/JCO.2024.42.17_suppl.LBA8505
U.S. Food and Drug Administration. FDA grants accelerated approval to sunvozertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations. Silver Spring (MD): FDA; 2025 [cited 2025 Dec 29].
Yang JCH, Wang M, Doucet L, Fan Y, Lv D, Sun M, et al. Phase II dose-randomized study of sunvozertinib in platinum-pretreated non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations (WU-KONG1B). J Clin Oncol. 2025;43(29):3198–208.
https://doi.org/10.1200/JCO-25-00788
U.S. Food and Drug Administration. FDA approves taletrectinib for ROS1-positive non-small cell lung cancer. Silver Spring (MD): FDA; 2025 [cited 2025 Dec 29].
U.S. Food and Drug Administration. FDA grants accelerated approval to zongertinib for non-squamous NSCLC with HER2-TKD activating mutations. Silver Spring (MD): FDA; 2025 [cited 2025 Dec 29].
U.S. Food and Drug Administration. FDA grants accelerated approval to sevabertinib for non-squamous non-small cell lung cancer. Silver Spring (MD): FDA; 2025 [cited 2025 Dec 29].
Ten Berge DMHJ, Damhuis RAM, Aerts JGJV, Dingemans AMC. Real-world treatment patterns and survival of patients with ROS1 rearranged stage IV non-squamous NSCLC in the Netherlands. Lung Cancer. 2023;181:107253.
https://doi.org/10.1016/j.lungcan.2023.107253
Patil T, Simons E, Mushtaq R, Pacheco JM, Doebele RC, Bowles DW. Targeted therapies for ROS1-rearranged non-small cell lung cancer. Drugs Today (Barc). 2019;55(10):641–52.
https://doi.org/10.1358/dot.2019.55.10.3030646
Li W, Xiong A, Yang N, Fan H, Yu Q, Zhao Y, et al. Efficacy and safety of taletrectinib in Chinese patients with ROS1+ non-small cell lung cancer: the phase II TRUST-I study. J Clin Oncol. 2024;42(22):2660–70.
https://doi.org/10.1200/JCO.24.00731
Nagasaka M, Ohe Y, Zhou C, Choi CM, Yang N, Liu G, et al. TRUST-II: a global phase II study of taletrectinib in ROS1-positive non-small-cell lung cancer and other solid tumors. Future Oncol. 2023;19(2):123–35.
https://doi.org/10.2217/fon-2022-1059
Neal JW, Lovly CM. Personalized, genotype-directed therapy for advanced non-small cell lung cancer. UpToDate. Waltham (MA): UpToDate Inc.; 2025 [cited 2025 Dec 28].
Heymach JV, Opdam F, Barve M, Tu HY, Wu YL, Berz D, et al. HER2-selective tyrosine kinase inhibitor, zongertinib (BI 1810631), in patients with advanced/metastatic solid tumors with HER2 alterations: a phase Ia dose-escalation study. J Clin Oncol. 2025;43(11):1337–47.
https://doi.org/10.1200/JCO-24-01727
Le X, Kim TM, Loong HH, Prelaj A, Goh BC, Li L, et al. Sevabertinib in advanced HER2-mutant non-small-cell lung cancer. N Engl J Med. 2025;393(18):1819–32.
https://doi.org/10.1056/NEJMoa2511065
Vranic S, Gatalica Z. Tumor-type agnostic, targeted therapies make a new step forward: the first tumor-agnostic approval of a HER2-targeted therapy. Biomol Biomed. 2024;24(4):673–5.
https://doi.org/10.17305/bb.2024.10641
Vranić S, Bešlija S, Gatalica Z. Targeting HER2 expression in cancer: new drugs and new indications. Bosn J Basic Med Sci. 2021;21(1):1–4.
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2026 Krešimir Tomić, Semir Vranić

This work is licensed under a Creative Commons Attribution 4.0 International License.









