Enantiomer-specific ketorolac pharmacokinetics in young women, including pregnancy and postpartum period
DOI:
https://doi.org/10.17305/bjbms.2016.1515Keywords:
Pharmacokinetics, ketorolac, enantiomers, pregnancy, cesarean delivery, postpartumAbstract
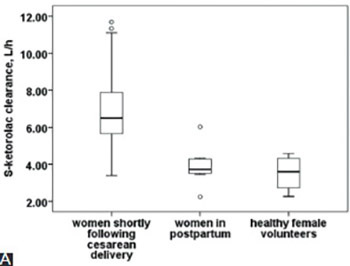
Racemic ketorolac clearance (CL) is significantly higher at delivery, but S-ketorolac disposition determines the analgesic effects. The aim of this study was to investigate the effect of pregnancy and postpartum period on enantiomer-specific (S and R) intravenous (IV) ketorolac pharmacokinetics (PKs). Data in women shortly following cesarean delivery (n=39) were pooled with data in a subgroup of these women that was reevaluated in the later postpartum period (postpartum group, n=8/39) and with eight healthy female volunteers. All women received single IV bolus of 30 mg ketorolac tromethamine. Five plasma samples were collected at 1, 2, 4, 6, and 8 hours and plasma concentrations were determined using high performance liquid chromatography. Enantiomer-specific PKs were calculated using PKSolver. Unpaired analysis showed that distribution volume at steady state (Vss, L/kg) for S- and R-ketorolac was significantly higher in women shortly following cesarean delivery (n=31) compared to postpartum group (n=8) or to healthy female volunteers (n=8). CL, CL to body weight, and CL to body surface area (CL/BSA) for S- and R-ketorolac were also significantly higher in women following delivery. In addition, S/R-ketorolac CL/BSA ratio was significantly higher at delivery. Paired PK analysis in eight women shortly following delivery and in postpartum group showed the same pattern. Finally, the simultaneous increase in CL and Vss resulted in similar estimates for elimination half-life in both unpaired and paired analysis. In conclusion, pregnancy affects S-, R-, and S/R-ketorolac disposition. This is of clinical relevance since S-ketorolac (analgesia) CL is even more increased compared to R-ketorolac CL, and S/R-ketorolac CL ratio is higher following delivery compared to postpartum period or to healthy female volunteers.
Citations
Downloads
References
Cohen MN, Christians U, Henthorn T, Vu Tran Z, Moll V, Zuk J, et al. Pharmacokinetics of single-dose intravenous ketorolac in infants aged 2-11 months. Anesth Analg 2011;112(3):655-60. http://dx.doi.org/10.1213/ANE.0b013e3182075d04.
Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet 1992;23(6):415-27.
http://dx.doi.org/10.2165/00003088-199223060-00003.
Mroszczak E, Combs D, Chaplin M, Tsina I, Tarnowski T, Rocha C, et al. Chiral kinetics and dynamics of ketorolac. J Clin Pharmacol 1996;36(6):521-39. http://dx.doi.org/10.1002/j.1552-4604.1996.tb05042.x.
Guzmán A, Yuste F, Toscano RA, Young JM, Van Horn AR, Muchowski JM. Absolute configuration of (-)-5-benzoyl-1,2-dihydro-3H-pyrrolo[1,2-alpha]pyrrole-1-carboxylic acid, the active enantiomer of ketorolac. J Med Chem 1986;29(4):589-91. http://dx.doi.org/10.1021/jm00154a027.
Jamali F. Pharmacokinetics of enantiomers of chiral non-steroidal anti-inflammatory drugs. Eur J Drug Metab Pharmacokinet 1988;13(1):1-9. http://dx.doi.org/10.1007/BF03189920.
Jung D, Mroszczak E, Bynum L. Pharmacokinetics of ketorolac tromethamine in humans after intravenous, intramuscular and oral administration. Eur J Clin Pharmacol 1988;35(4):423-5. http://dx.doi.org/10.1007/BF00561376.
Lapicque F, Muller N, Payan E, Dubois N, Netter P. Protein binding and stereoselectivity of nonsteroidal anti-inflammatory drugs. Clin Pharmacokinet 1993;25(2):115-23. http://dx.doi.org/10.2165/00003088-199325020-00004.
Kauffman RE, Lieh-Lai MW, Uy HG, Aravind MK. Enantiomer-selective pharmacokinetics and metabolism of ketorolac in children. Clin Pharmacol Ther 1999;65(4):382-8. http://dx.doi.org/10.1016/S0009-9236(99)70131-1.
Feghali MN, Mattison DR. Clinical therapeutics in pregnancy. J Biomed Biotechnol 2011;2011:783528. http://dx.doi.org/10.1155/2011/783528.
Lynn AM, Bradford H, Kantor ED, Andrew M, Vicini P, Anderson GD. Ketorolac tromethamine: Stereo-specific pharmacokinetics and single-dose use in postoperative infants aged 2-6 months. Paediatr Anaesth 2011;21(3):325-34. http://dx.doi.org/10.1111/j.1460-9592.2010.03484.x.
Hamunen K, Maunuksela EL, Sarvela J, Bullingham RE, Olkkola KT. Stereoselective pharmacokinetics of ketorolac in children, adolescents and adults. Acta Anaesthesiol Scand 1999;43(10):1041-6. http://dx.doi.org/10.1034/j.1399-6576.1999.431012.x.
Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011;377(9784):2215-25. http://dx.doi.org/10.1016/S0140-6736(11)60245-6.
McDonnell NJ, Keating ML, Muchatuta NA, Pavy TJ, Paech MJ. Analgesia after caesarean delivery. Anaesth Intensive Care 2009;37(4):539-51.
Pan PH. Post cesarean delivery pain management: Multimodal approach. Int J Obstet Anesth 2006;15(3):185-8. http://dx.doi.org/10.1016/j.ijoa.2006.04.004.
White PF, Raeder J, Kehlet H. Ketorolac: Its role as part of a multimodal analgesic regimen. Anesth Analg 2012;114(2):250-4. http://dx.doi.org/10.1213/ANE.0b013e31823cd524.
Pavy TJ, Paech MJ, Evans SF. The effect of intravenous ketorolac on opioid requirement and pain after cesarean delivery. Anesth Analg 2001;92(4):1010-4. http://dx.doi.org/10.1097/00000539-200104000-00038.
Dahl V, Hagen IE, Sveen AM, Norseng H, Koss KS, Steen T. High-dose diclofenac for postoperative analgesia after elective caesarean section in regional anaesthesia. Int J Obstet Anesth 2002;11(2):91-4. http://dx.doi.org/10.1054/ijoa.2001.0931.
De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: A meta-analysis of randomized trials. Anesth Analg 2012;114(2):424-33. http://dx.doi.org/10.1213/ANE.0b013e3182334d68.
Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 2009;48(3):143-57.
http://dx.doi.org/10.2165/00003088-200948030-00001.
Messing K, Mager Stellman J. Sex, gender and women’s occupational health: The importance of considering mechanism. Environ Res 2006;101(2):149-62. http://dx.doi.org/10.1016/j.envres.2005.03.015.
Kulo A, van de Velde M, van Calsteren K, Smits A, de Hoon J, Verbesselt R, et al. Pharmacokinetics of intravenous ketorolac following caesarean delivery. Int J Obstet Anesth 2012;21(4):334-8. http://dx.doi.org/10.1016/j.ijoa.2012.06.001.
Hebert MF. Impact of pregnancy on pharmacokinetics of medications. J Popul Ther Clin Pharmacol 2013;20(3):350-7.
Kulo A, Hendrickx S, de Hoon J, Mulabegovic N, van Calsteren K, Verbesselt R, et al. The impact of pregnancy on urinary ketorolac metabolites after single intravenous bolus. Eur J Drug Metab Pharmacokinet 2013;38(1):1-4.
http://dx.doi.org/10.1007/s13318-012-0108-7.
Allegaert K, Kulo A, Verbesselt R, Hopchet L, Deprest J, de Hoon J, et al. The pharmacokinetics of a high intravenous dose of paracetamol after caesarean delivery: The effect of gestational age. Eur J Anaesthesiol 2012;29(10):484-8. http://dx.doi.org/10.1097/EJA.0b013e32835543a0.
Kulo A, Mulabegovic N, Loga-Zec S, Allegaert K, de Hoon J, Verbesselt R. Determination of racemic ketorolac, ketorolac enantiomers and their metabolites in human plasma and urine by LC–UV, applied in clinical study during and after pregnancy. Chromatographia 2014;77(11):803-12. http://dx.doi.org/10.1007/s10337-014-2670-4.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 2010;99(3):306-14. http://dx.doi.org/10.1016/j.cmpb.2010.01.007.
Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J Pediatr 1978;93(1):62-6. http://dx.doi.org/10.1016/S0022-3476(78)80601-5.
Allegaert K, Van Mieghem T, Verbesselt R, Vanhole C, Devlieger R, Cossey V, et al. Cefazolin plasma protein binding saturability during pregnancy. Methods Find Exp Clin Pharmacol 2009;31(1):25-8. http://dx.doi.org/10.1358/mf.2009.31.1.1346611.
Koren G. Sex dependent pharmacokinetics and bioequivalence – Time for a change. J Popul Ther Clin Pharmacol 2013;20(3):e358-61.
Allegaert K, van Calsteren K, Hendrickx S, Kelchtermans J, Smits A, Kulo A, et al. Paracetamol and ketorolac pharmacokinetics and metabolism at delivery and during postpartum. Acta Anaesthesiol Belg 2012;63(3):121-5.
Kulo A, van Calsteren K, Verbesselt R, Smits A, Devlieger R, de Hoon J, et al. The impact of Caesarean delivery on paracetamol and ketorolac pharmacokinetics: A paired analysis. J Biomed Biotechnol 2012;2012:437639. http://dx.doi.org/10.1155/2012/437639.
Gravenstein D, Suri A, Derendorf HC, Koska AJ. Influence of plasma expansion on plasma protein binding of ketorolac. J Clin Anesth 1998;10(6):464-8. http://dx.doi.org/10.1016/S0952-8180(98)00064-6.
Mroszczak EJ, Lee FW, Combs D, Sarnquist FH, Huang BL, Wu AT, et al. Ketorolac tromethamine absorption, distribution, metabolism, excretion, and pharmacokinetics in animals and humans. Drug Metab Dispos 1987;15(5):618-26.
Olkkola KT, Maunuksela EL. The pharmacokinetics of postoperative intravenous ketorolac tromethamine in children. Br J Clin Pharmacol 1991;31(2):182-4. http://dx.doi.org/10.1111/j.1365-2125.1991.tb05510.x.
Hayball PJ, Wrobel J, Tamblyn JG, Nation RL. The pharmacokinetics of ketorolac enantiomers following intramuscular administration of the racemate. Br J Clin Pharmacol 1994;37(1):75-8. http://dx.doi.org/10.1111/j.1365-2125.1994.tb04243.x.
Kulo A, Peeters MY, Allegaert K, Smits A, de Hoon J, Verbesselt R, et al. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. Br J Clin Pharmacol 2013;75(3):850-60. http://dx.doi.org/10.1111/j.1365-2125.2012.04402.x.
Mohammed BS, Engelhardt T, Hawwa AF, Cameron GA, McLay JS. The
enantioselective population pharmacokinetics of intravenous ketorolac in children
using a stereoselective assay suitable for microanalysis. J Pharm Pharmacol 2015;67(9):1179-87. http://dx.doi.org/10.1111/jphp.12418.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-09-27
Published 2017-02-21









