Atypical sympathomimetic drug lerimazoline mediates contractile effects in rat aorta predominantly by 5-HT2A receptors
DOI:
https://doi.org/10.17305/bjbms.2017.2071Keywords:
Lerimazoline, rat aorta, binding affinity, phenylephrine, 5-HT2A receptors, St-71, trimizoline, tramazoline, antagonist activity, sympathomimetic drugAbstract
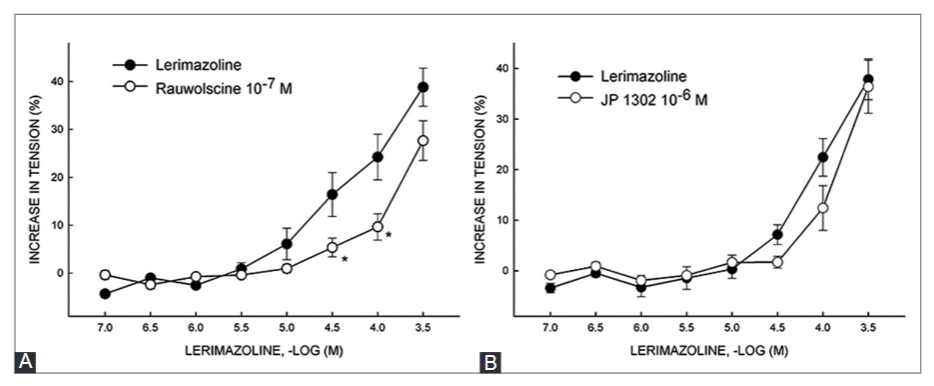
Lerimazoline is a sympathomimetic drug that belongs to the imidazoline class of compounds, and is used as a nasal decongestant. Studies on lerimazoline are rare, and its pharmacological profile is not completely understood. Here, we analyzed the affinity of lerimazoline for dopamine receptor D2, serotonin 5-HT1A and 5-HT2A receptors and α1-adrenoceptor, and investigated lerimazoline contractile effects in isolated rat thoracic aorta. We also determined the effect of several antagonists on the contractile response to lerimazoline, including prazosin (α1-adrenoceptor antagonist), RX 821002 and rauwolscine (α2-adrenoceptor antagonists), JP 1302 (α2C-adrenoceptor antagonist), methiothepin (non-selective 5-HT receptor antagonist), SB 224289 (5-HT1B receptor antagonist), BRL 15572 (5-HT1D receptor antagonist), and ketanserin (5-HT2A receptor antagonist). Lerimazoline displayed high affinity for the 5-HT1A receptor (Ki = 162.5 nM), similar to the previously reported affinity for the 5-HT1D receptor. Binding affinity estimates (Ki) for α1, 5-HT2A, and D2 receptors were 6656, 4202 and 3437.5 nM, respectively (the literature reported Ki for 5-HT1B receptor is 3480 nM). Lerimazoline caused concentration-dependent contractions in 70% of preparations, varying in the range between 40% and 55% of the maximal contraction elicited by phenylephrine. While prazosin reduced the maximum contractile response to lerimazoline, rauwolscine showed a non-significant trend in reduction of the response. Both ketanserin (10 nM and 1 µM) and methiothepin strongly suppressed the maximum response to lerimazoline. Overall, our results suggest that 5-HT2A and, less distinctly, α1-adrenergic receptors are involved in the lerimazoline-induced contractions, which makes lerimazoline an “atypical” decongestant.
Citations
Downloads
References
Johnson DA, Hricik JG. The pharmacology of α-adrenergic decongestants. Pharmacotherapy 1993;13(6 Pt 2):110S-5S.
Mortuaire G, de Gabory L, François M, Massé G, Bloch F, Brion N, et al. Rebound congestion and rhinitis medicamentosa: Nasal decongestants in clinical practice. Critical review of the literature by a medical panel. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130(3):137-44. https://doi.org/10.1016/j.anorl.2012.09.005.
Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al. Automated design of ligands to polypharmacological profiles. Nature 2012;492(7428):215-20. https://doi.org/10.1038/nature11691.
Peters JU. Polypharmacology - foe or friend? J Med Chem 2013;56(22):8955-71. https://doi.org/10.1021/jm400856t.
Christ GJ, Jean-Jacques M. Mutual-effect amplification of contractile responses elicited by simultaneous activation of alpha-1 adrenergic and 5-hydroxytryptamine2 receptors in isolated rat aorta. J Pharmacol Exp Ther 1991;256(2):553-61.
Law H, Dukat M, Teitler M, Lee DK, Mazzocco L, Kamboj R, et al. Benzylimidazolines as h5-HT1B/1D serotonin receptor ligands: A structure-affinity investigation. J Med Chem 1998;41(13):2243-51. https://doi.org/10.1021/jm970513p.
Schoeffter P, Hoyer D. Interaction of the alpha-adrenoceptor agonist oxymetazoline with serotonin 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors. Eur J Pharmacol 1991;196(2):213-6. https://doi.org/10.1016/0014-2999(91)90432-P.
Huang XP, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, et al. Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine(2B) receptor agonists: Implications for drug safety assessment. Mol Pharmacol 2009;76(4):710-22. https://doi.org/10.1124/mol.109.058057.
Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease - it was meant 2B. Pharmacol Ther 2011;132(2):146-57. https://doi.org/10.1016/j.pharmthera.2011.03.008.
Uddman R, Longmore J, Cardel LO, Edvinsson L. Expression of 5-HT1B receptors in human nasal mucosa. Acta Otolaryngol 2001;121(3):403-6. https://doi.org/10.1080/000164801300102932.
World Health Organization (WHO). International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 72: Lerimazoline. WHO Drug Information [Internet]. [cited 2016 Apr 17] 28(3):400.2014. Available from: http://apps.who.int/medicinedocs/documents/s21577en/s21577en.pdf.
Zeile K, Hauptmann K, Staehle H, inventors; Boehringer Sohn Ingelheim, assignee. Verfahren zur Herstellung von 2-(2', 4', 6'-Trimethylbenzyl)-1, 3-diazacyclopenten-(2) und seinen Salzen. German patent DE1154119. 1963 Sep 12.
Brayfield A. Martindale: The complete drug reference. 38th ed. London: Pharmaceutical Press; 2014.
Malta E, Ong JS, Raper C, Tawa PE, Vaughan GN. Structure activity relationships of clonidine- and tolazoline-like compounds at histamine and α-adrenoceptor sites. Br J Pharmacol 1980;69(4):679-88. https://doi.org/10.1111/j.1476-5381.1980.tb07922.x.
Malta E, Raper C, Tawa PE. Pre- and postjunctional effects of clonidine- and oxymetazoline-like compounds in guinea-pig ileal preparations. Br J Pharmacol 1981;73(2):355-62. https://doi.org/10.1111/j.1476-5381.1981.tb10429.x
Nathanson JA. Phenyliminoimidazolidines. Characterization of a class of potent agonists of octopamine-sensitive adenylate cyclase and their use in understanding the pharmacology of octopaminereceptors. Mol Pharmacol 1985;28(3):254-68.
Boudier HS, de Boer J, Smeets G, Lien EJ, van Rossum J. Structure activity relationship for central and peripheral alpha adrenergic activities of imidazoline derivatives. Life Sci 1975;17(3): 377-85. https://doi.org/10.1016/0024-3205(75)90487-7.
Selli Ç, Eraç Y, Tosun M. Effects of 1-(2-trifluoromethylphenyl)-imidazole (TRIM) on receptor-independent and -dependent contractile responses in rat aorta. Turk J Med Sci 2016;46(4):1209-14. https://doi.org/10.3906/sag-1502-109.
Rameshrad M, Babaei H, Azarmi Y, Fouladia DF. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci 2016;145:190-204. https://doi.org/10.1016/j.lfs.2015.12.043.
Tomić M, Kundaković M, Butorović B, Vasilev V, Dragović D, Roglić G, et al. Pharmacological evaluation of 5-{2-[4-(2-methox-yphenyl)-piperazin-1-yl]ethyl}-1,3-dihydro-benzimidazole-2-thione as a potential atypical antipsychotic agent. Pharmazie 2003;58:677-8.
Flavahan NA, McGrath JC. Are human vascular α-adrenoceptors atypical? J Cardiovasc Pharmacol 1984;6:208-10.
Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 2000;278(4):H1075-83.
Corboz MR, Rivelli MA, Varty L, Mutter J, Cartwright M, Rizzo CA, et al. Pharmacological characterization of postjunctional α-adrenoceptors in human nasal mucosa. Am J Rhinol 2005;19(5):495-502.
Honner V, Docherty JR. Investigation of the subtypes of α1-adrenoceptor mediating contractions of rat vas deferens. Br J Pharmacol 1999;128(6):1323-31. https://doi.org/10.1038/sj.bjp.0702913.
McKune CM, Watts SW. Characterization of the serotonin receptor mediating contraction in the mouse thoracic aorta and signal pathway coupling. J Pharmacol Exp Ther 2001;297(1):88-95.
Martin GR. Vascular receptors for 5-hydroxytriptamine: Distribution, function and classification. Phamacol Ther 1994;62(3):283-324.
https://doi.org/10.1016/0163-7258(94)90048-5.
Bhattacharya A, Schenck KW, Xu YC, Nisenbaum L, Galbreath E, Cohen ML. 5-Hydroxytryptamine1B receptor-mediated contraction of rabbit saphenous vein and basilar artery: Role of vascular endothelium. J Pharmacol Exp Ther 2004;309(2):825-32. https://doi.org/10.1124/jpet.103.062653.
Gul H, Yildiz O. Amplification of sumatriptan-induced contractions with phenylephrine, histamine and KCl in the isolated human mesenteric artery: In-vitro evidence for sumatriptan-induced mesenteric ischemia. Naunyn Schmiedebergs Arch Pharmacol 2002;366(3):254-61. https://doi.org/10.1007/s00210-002-0587-1.
Doxey JC, Lane AC, Roach AG, Virdee NK. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch Pharmacol 1984;325(2):136-44. https://doi.org/10.1007/BF00506193.
Doménech T, Beleta J, Palacios JM. Characterization of human serotonin 1D and 1B receptors using [3H]-GR-125743, a novel radiolabelled serotonin 5HT1D/1B receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol 1997;356(3):328-34. https://doi.org/10.1007/PL00005058.
Yoshio R, Taniguchi T, Itoh H, Muramatsu I. Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn J Pharmacol 2001;86(2): 189-95. https://doi.org/10.1254/jjp.86.189.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2017 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2017-05-12
Published 2017-08-20









