Etoricoxib improves osteoarthritis pain relief, joint function, and quality of life in the extreme elderly
DOI:
https://doi.org/10.17305/bjbms.2017.2214Keywords:
Pain, extreme elderly, osteoarthritis, etoricoxib, joint function, quality of lifeAbstract
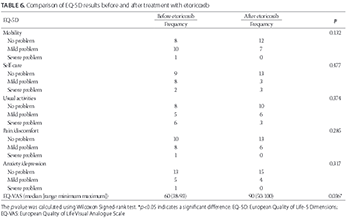
Etoricoxib is a selective cyclooxygenase-2 inhibitor, with a lower risk of gastrointestinal toxicity compared to traditional nonsteroidal anti-inflammatory drugs (NSAIDs). We evaluated the effectiveness and tolerability of etoricoxib in extremely elderly patients with chronic pain due to osteoarthritis (OA). A prospective, single-center, single-arm study was conducted, enrolling 19 extremely elderly men with OA (mean age 85.9, range 79-96 years), who responded inadequately to NSAIDs or other analgesics. Patients were switched to etoricoxib, 60 mg once daily for 4 weeks, without prior medication washout. Data were recorded before and after etoricoxib treatment. The primary endpoint was improvement in pain, assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) after the 4-week treatment. Other endpoints included the Brief Pain Inventory Short Form (BPI-SF), Treatment Satisfaction Questionnaire for Medication (TSQM), Short Form 36 (SF36), and European Quality of Life-5 Dimensions (EQ-5D). Safety and tolerability were assessed by collecting adverse events data. Pain and disability scores measured by WOMAC index were lower after treatment (pain, p ≤ 0.001; disability, p = 0.020). BPI-SF showed a significant improvement in joint function when walking and performing normal work (walking, p = 0.021; normal work, p = 0.030). SF36 scores improved for 7 out of 11 items after etoricoxib treatment (#1, p = 0.032; #4, p = 0.026; #5, p = 0.017; #6, p = 0.008; #7, p = 0.009; #8, p = 0.013; and #10, p = 0.038). EQ-5D showed a significant improvement in visual analogue scale scores (p = 0.036). TSQM results demonstrated a higher patient perception of overall satisfaction. No adverse events were reported. Pain relief, joint function, quality of life, and treatment satisfaction improved significantly in elderly patients with OA after etoricoxib administration.
Citations
Downloads
References
Fine PG. Chronic pain management in older adults: Special considerations. J Pain Symptom Manage 2009;38(2 Suppl):S4-S14. DOI: 10.1016/j.jpainsymman.2009.05.002.
Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: A prospective representative survey. Acta Anaesthesiol Scand 2008;52(1):132-6. https://doi.org/10.1111/j.1399-6576.2007.01486.x.
AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002;50(6 Suppl):S205-24.
Savage R. Cyclo-oxygenase-2 inhibitors: When should they be used in the elderly? Drugs Aging 2005;22(3):185-200. https://doi.org/10.2165/00002512-200522030-00001.
Brooks PM. Impact of osteoarthritis on individuals and society: How much disability? Social consequences and health economic implications. Curr Opin Rheumatol 2002;14(5):573-7. https://doi.org/10.1097/00002281-200209000-00017.
Puopolo A, Boice JA, Fidelholtz JL, Littlejohn TW, Miranda P, Berrocal A, et al. A randomized placebo-controlled trial comparing the efficacy of etoricoxib 30 mg and ibuprofen 2400 mg for the treatment of patients with osteoarthritis. Osteoarthritis Cartilage 2007;15(12):1348-56. https://doi.org/10.1016/j.joca.2007.05.022.
Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet 1999;354(9186):1248-52.
https://doi.org/10.1016/S0140-6736(99)03057-3.
McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med 2010;26(3):387-99. https://doi.org/10.1016/j.cger.2010.04.001.
Zambon S, Siviero P, Denkinger M, Limongi F, Victoria Castell M, van der Pas S, et al. Role of osteoarthritis, comorbidity, and pain in determining functional limitations in older populations: European project on osteoarthritis. Arthritis Care Res (Hoboken) 2016;68(6):801-10. https://doi.org/10.1002/acr.22755.
Breedveld FC. Osteoarthritis-the impact of a serious disease. Rheumatology (Oxford) 2004;43(Suppl 1):i4-i8. https://doi.org/10.1093/rheumatology/keh102.
Woolf AD, Pflieger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81(9):646-56.
Landi F, Russo A, Liperoti R, Danese P, Maiorana E, Pahor M, et al. Daily pain and functional decline among old-old adults living in the community: Results from the ilSIRENTE Study. J Pain Symptom Manage 2009;38(3):350-7. https://doi.org/10.1016/j.jpainsymman.2008.10.005.
Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, et al. Osteoarthritis: New insights. Part 2: Treatment approaches. Ann Intern Med 2000;133(9):726-37. https://doi.org/10.7326/0003-4819-133-9-200011070-00015.
Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;2:CD004257. https://doi.org/10.1002/14651858.CD004257.
Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. American College of Rheumatology. Arthritis Rheum 1995;38(11):1535-40. https://doi.org/10.1002/art.1780381103.
Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American college of rheumatology. Arthritis Rheum 1995;38(11):1541-6. https://doi.org/10.1002/art.1780381104.
Bakhriansyah M, Souverein PC, de Boer A, Klungel OH. Gastrointestinal toxicity among patients taking selective COX-2 inhibitors or conventional NSAIDs, alone or comined with proton pump inhiitors: A case-control study. Pharacoepidemiol Drug Saf 2017;26(10):1141-1148. https://doi.org/10.1002/pds.4183.
Cochrane DJ, Jarvis B, Keating GM. Etoricoxib. Drugs 2002;62(18):2637-51. https://doi.org/10.2165/00003495-200262180-00006.
Cannon CP, Curtis SP, FitzGerald GA, Krum H, Kaur A, Bolognese JA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the multinational etoricoxib and diclofenac arthritis long-term (MEDAL) programme: A randomised comparison. Lancet 2006;368(9549):1771-81. https://doi.org/10.1016/S0140-6736(06)69666-9.
Hunt RH, Harper S, Callegari P, Yu C, Quan H, Evans J, et al. Complementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxib. Aliment Pharmacol Ther 2003;17(2):201-10. https://doi.org/10.1046/j.1365-2036.2003.01407.x.
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352(11):1092-102. https://doi.org/10.1056/NEJMoa050493.
Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006;355(9):873-84. https://doi.org/10.1056/NEJMoa061355.
Denman M. Etoricoxib was noninferior to diclofenac for cardiovascular outcomes in osteoarthritis and rheumatoid arthritis. ACP J Club 2007;146(2):44.
Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332(7553):1302-8. https://doi.org/10.1136/bmj.332.7553.1302.
Lin HY, Cheng TT, Wang JH, Lee CS, Chen MH, Lei V, et al. Etoricoxib improves pain, function and quality of life: Results of a real-world effectiveness trial. Int J Rheum Dis 2010;13(2):144-50. https://doi.org/10.1111/j.1756-185X.2010.01468.x.
Croom KF, Siddiqui MA. Etoricoxib: A review of its use in the symptomatic treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and acute gouty arthritis. Drugs 2009;69(11):1513-32. https://doi.org/10.2165/00003495-200969110-00008.
Leung AT, Malmstrom K, Gallacher AE, Sarembock B, Poor G, Beaulieu A, et al. Efficacy and tolerability profile of etoricoxib in patients with osteoarthritis: A randomized, double-blind, placebo and active-comparator controlled 12-week efficacy trial. Curr Med Res Opin 2002;18(2):49-58. https://doi.org/10.1185/030079902125000282.
Gottesdiener K, Schnitzer T, Fisher C, Bockow B, Markenson J, Ko A, et al. Results of a randomized, dose-ranging trial of etoricoxib in patients with osteoarthritis. Rheumatology (Oxford) 2002;41(9):1052-61. https://doi.org/10.1093/rheumatology/41.9.1052.
Hunt RH, Harper S, Watson DJ, Yu C, Quan H, Lee M, et al. The gastrointestinal safety of the COX-2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal events. Am J Gastroenterol 2003;98(8):1725-33.
https://doi.org/10.1111/j.1572-0241.2003.07598.x.
Zacher J, Feldman D, Gerli R, Scott D, Hou SM, Uebelhart D, et al. A comparison of the therapeutic efficacy and tolerability of etoricoxib and diclofenac in patients with osteoarthritis. Curr Med Res Opin 2003;19(8):725-36. https://doi.org/10.1185/030079903125002469.
Wiesenhutter CW, Boice JA, Ko A, Sheldon EA, Murphy FT, Wittmer BA, et al. Evaluation of the comparative efficacy of etoricoxib and ibuprofen for treatment of patients with osteoarthritis: A randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 2005;80(4):470-9. https://doi.org/10.4065/80.4.470.
Bingham CO 3rd, Sebba AI, Rubin BR, Ruoff GE, Kremer J, Bird S, et al. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford) 2007;46(3):496-507. https://doi.org/10.1093/rheumatology/kel296.
Reginster JY, Malmstrom K, Mehta A, Bergman G, Ko AT, Curtis SP, et al. Evaluation of the efficacy and safety of etoricoxib compared with naproxen in two, 138-week randomised studies of patients with osteoarthritis. Ann Rheum Dis 2007;66(7):945-51. https://doi.org/10.1136/ard.2006.059162.
Baraf HS, Fuentealba C, Greenwald M, Brzezicki J, O’Brien K, Soffer B, et al. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: Results of the etoricoxib versus diclofenac sodium gastrointestinal tolerability and effectiveness (EDGE) trial. J Rheumatol 2007;34(2):408-20.
Dobre D, van Veldhuisen DJ, DeJongste MJ, van Sonderen E, Klungel OH, Sanderman R, et al. The contribution of observational studies to the knowledge of drug effectiveness in heart failure. Br J Clin Pharmacol 2007;64(4):406-14.
https://doi.org/10.1111/j.1365-2125.2007.03010.x.
Ioannidis JP, Haidich AB, Lau J. Any casualties in the clash of randomised and observational evidence? BMJ 2001;322(7291):879-80. https://doi.org/10.1136/bmj.322.7291.879.
Matsumoto AK, Cavanaugh PF Jr. Etoricoxib. Drugs Today (Barc) 2004;40(5):395-414. https://doi.org/10.1358/dot.2004.40.5.850488.
World Health Organization. Current Status of the World Health Survey. Geneva, Switzerland: WHO International; 2011. Available from: http://www.who.int. [Last accessed on 2014 Mar 10].
Brodsky J, Habib J, Mizrahi I. Long-Term Care Laws in Five Developed Countries: A Review. Jerusalem: Brookdale Institute of Gerontology and Human Development; 2002.
Matsumoto A, Melian A, Shah A, Curtis SP. Etoricoxib versus naproxen in patients with rheumatoid arthritis: A prospective, randomized, comparator-controlled 121-week trial. Curr Med Res Opin 2007;23(9):2259-68. https://doi.org/10.1185/030079907X219625.
Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: A systematic review and economic evaluation. Health Technol Assess 2008;12(11):1-278, iii.
Etoricoxib: New drug. Avoid using cox-2 inhibitors for pain. Prescrire Int 2007;16(92):223-7.
Yaksh TL, Woller SA, Ramachandran R, Sorkin LS. The search for novel analgesics: Targets and mechanisms. F1000Prime Rep 2015;7:56. https://doi.org/10.12703/P7-56.
Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res 2007;5(1):19-34. https://doi.org/10.3121/cmr.2007.698.
Atkinson TJ, Fudin J, Jahn HL, Kubotera N, Rennick AL, Rhorer M. What’s new in NSAID pharmacotherapy: Oral agents to injectables. Pain Med 2013;14(Suppl 1):S11-7. https://doi.org/10.1111/pme.12278.
Yaksh T, Wallace MS. Opioids, analgesia, and pain management. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill Medical; 2011. p. 481-526.
Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med 2010;16(11):1241-7. https://doi.org/10.1038/nm.2230.
Kissin I. The development of new analgesics over the past 50 years: A lack of real breakthrough drugs. Anesth Analg 2010;110(3):780-9. https://doi.org/10.1213/ANE.0b013e3181cde882.
Laufer S. Osteoarthritis therapy-are there still unmet needs? Rheumatology (Oxford) 2004;43(Suppl 1):i9-i15. https://doi.org/10.1093/rheumatology/keh103.
Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969;9(1):179-86. https://doi.org/10.1093/geront/9.3_Part_1.179.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-08-14
Published 2018-02-20









