B-type natriuretic peptide and adiponectin releases in rat model of myocardial damage induced by isoproterenol administration
DOI:
https://doi.org/10.17305/bjbms.2013.2329Keywords:
adiponectin, isoproterenol, B-type natriuretic peptide, myocardial damageAbstract
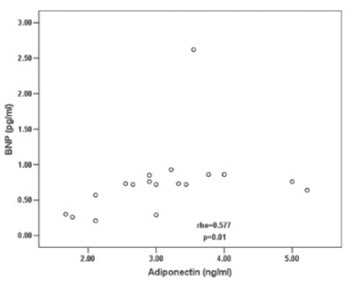
B-type natriuretic peptide (BNP) and adiponectin play important role in the cardiovascular homeostasis regulation. We investigated BNP and adiponectin serum levels followed by isoproterenol (ISO) administration to rats and explored the relationship between them. Cardiac troponin I (cTnI) blood level was used as biochemical evidence of myocardial damage development. Adult male Wistar rats (average body weight 273.33 ± 21.63 g) were distributed into groups: control group received saline (n=6) and ISO groups (n=12) treated with ISO (subcutaneous single dose 100 mg/kg of rat body weight). ISO group was divided into two groups according to the time of BNP, adiponectin and cTnI determination: ISO I (n=6; 2 hours after ISO administration); ISO II (n=6; 4 hours after ISO administration). Blood for determination of parameters was taken from rat abdominal aorta. BNP, adiponectin and cTnI were determined by ELISA method. Data were statistically analysed by using SPSS version 13 computer program. P value less 0.05 was considered statistically significant. Blood BNP and adiponectin were lower at 2 hours after ISO administration in comparison with control group (p=0.004 for BNP and p=0.174 for adiponectin). Four hours after ISO administration, we have noted significant elevation of both parameters compared to ISO I group (p=0.004 for BNP; p=0.02 for adiponectin). Test of correlation have showed significant relation between their blood levels during experimental period (rho=0.577; p=0.01). BNP and adiponectin are not simple indicators of myocardial damage development. They have possible associated and additive effects in cardiovascular homeostasis regulation.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
How to Cite
Accepted 2017-08-07
Published 2013-11-20









