Induction of apoptosis by grape seed extract (Vitis vinifera) in oral squamous cell carcinoma
DOI:
https://doi.org/10.17305/bjbms.2013.2360Keywords:
cytotoxicity, apoptosis, Anticancer, oral squamous cell carcinoma, grape seed extractAbstract
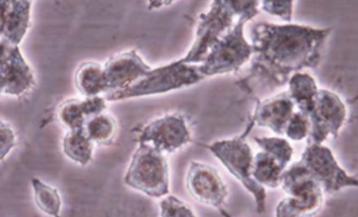
Development of novel therapeutic modalities is crucial for the treatment of oral squamous cell carcinoma (OSCC). Recent scientific studies have been focused on herbal medicines as potent anti-cancer drug candidates. This study is the first to investigate the cytotoxic effects and the mechanism of cell death induced by grape seed extract (GSE) in oral squamous cell carcinoma (KB cells). MTT (3-(4,5-dimetylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) and trypan blue assays were performed in KB cells as well as human umbilical vein endothelial cells (HUVEC) were used to analyze the cytotoxic activity of GSE. Furthermore, the apoptosis-inducing action of the extract was determined by TUNEL, DNA fragmentation and cell death analysis. Statistical significance was determined by analysis of variance (ANOVA), followed by Duncan’s test at a significance level of P≤0.05. The results showed apoptotic potential of GSE, confirmed by significant inhibition of cell growth and viability in a dose- and time- dependent manner without inducing damage to non-cancerous cell line HUVEC. The results of this study suggest that this plant contains potential bioactive compound(s) for the treatment of oral squamous cell carcinoma.
Citations
Downloads
Downloads
Additional Files
Published
License
Copyright (c) 2017 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2017-08-13
Published 2013-08-20









