Heart-type fatty acid-binding protein and its relation with morphological changes in rat myocardial damage model induced by isoproterenol
DOI:
https://doi.org/10.17305/bjbms.2011.2557Keywords:
H-FABP, isoproterenol, histology, myocardial lesion score, ratAbstract
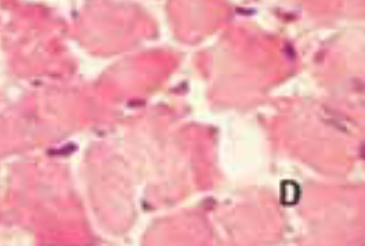
We have investigated heart type fatty acid binding protein (H-FABP) rat serum values at different time point following subcutaneous (s.c) isoproterenol (ISO) administration and their correlation with severity of myocardial lesion. Thirty adult, male, Wistar rats were used for this study. Six rats per group were treated with a single dose of either ISO (ISO groups, dose 100 mg/kg, s.c.) at different time point (30’, 60’, 120’, 240’) or with saline (control group). Serum H-FABP was determined by enzyme-linked immunosorbent assay (ELISA) and histological analysis was performed by hematoxylin-eosin (HE) method of staining. The first serum H-FABP increase was obtained 30’ following ISO administration, but maximal value was reached after 240’. Myocardial histological changes were time-dependent and correlated with serum H-FABP values (p<0.001). The results of the study suggest that H-FABP is sensitive marker for acute rat myocardial injury and its possible inclusion in myocardial injury screening studies in rats.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2017 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2017-10-12
Published 2011-11-20









