Investigation of Ivs14+1G>A Polymorphism of Dpyd Gene in a Group of Bosnian Patients Treated with 5-Fluorouracil and Capecitabine
DOI:
https://doi.org/10.17305/bjbms.2010.2712Keywords:
pharmacogenetics, Dihydropyrimidine dehydrogenase, DPYD2A mutationAbstract
Adverse drug reactions still pose an important clinical problem. Dihydropyrimidine dehydrogenase (DPD) is an enzyme that regulates 5-FU quantities available for anabolic processes and hence affects its pharmacokinetics, toxicity and efficacy.
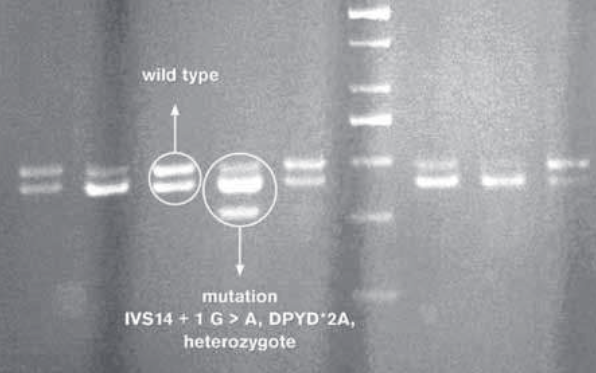
There are several studies describing a hereditary (pharmacogenetic) disorder in which individuals with absent or significantly reduced DPD activity may even develop a life-threatening toxicity following exposure to 5-FU. The most common mutation is known as the DPYD*2A or as the splice-site mutation (IVS14 + 1G A) leading to creation of a dysfunctional protein. An objective behind the study was to ascertain existence of the IVS14+ 1G A mutation among the population of Bosnia and Herzegovina. Our research has undeniably attested to existence of one heterozygote for the DPYD gene mutation, i.e. one heterozygote for IVS14 + 1 G > A, DPYD*2A mutation.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2017 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2017-11-24
Published 2010-05-20









