Oral acute toxicity of HEPALIP FORTE in rats
DOI:
https://doi.org/10.17305/bjbms.2003.3489Keywords:
acute toxicity, essential phospholipidsAbstract
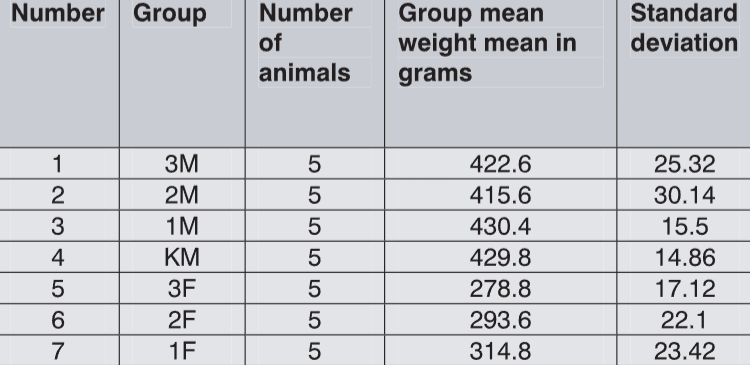
he main active component of preparation HEPALIP FORTE is EPL--essential phospholipids. Their chemical structure corresponds to that of endogen phospholipids, but they have functional superiority because of the content of unsaturated fatty acids. Essential phospholipids in combination with the vitamins have been used in the treatment of liver diseases, dyslipoproteinaemias and intoxications with consequent liver failure. Acute toxicity study on HEPALIP FORTE was performed on Wistar rats. The main aim of toxicology studies for the drug registration process is evaluation of the toxic potential and risks of human exposition to the substance (Gelbke et al., 1999). Acute toxicity is an orientation point of the test substance toxicity and represents a starting test for the toxicological evaluation. Study included one oral dose of the substance, applied with oesophageal intubations. There were three dose-levels: 300, 500 and 1000 mg/kg. No lethality was recorded and statistical analysis of body weight variations failed to show any significant difference between the groups. Reversible tremor was more frequently recorded in females and was not present in control animals. After the planed sacrifice, no changes related to the test substance were recorded. We noticed a statistically significant difference in the liver weights between males of 3M and 2M groups in comparison to the control. Similar (not significant) tendency was noticed in females. Significant differences in organ weights might be suggestive of a toxic effect that experimental animal managed to recover from in partial manner. The histopathological analysis detected no changes in the structure and morphology of liver parenchyma.
Citations
Downloads
Downloads
Published
License
Copyright (c) 2018 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2018-04-13
Published 2003-11-20









