The new trends in theophylline therapy
DOI:
https://doi.org/10.17305/bjbms.2002.3584Keywords:
theophylline, sustained-release, pellets, asthma, theophylline serum concentrations, lung function parameters, once-daily evening administrationAbstract
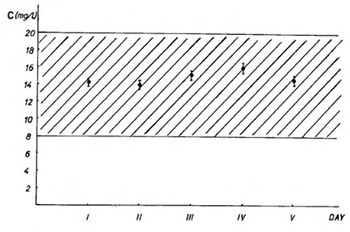
Sustained-release theophylline pellets formulation for once-daily evening administration significantly improved patients compliance and adjusted serum levels profile of the drug. The patients conversion from i.v. to p.o. therapy is one of the most critical steps in the treatment of asthma according to its chronopathophysiological character. In our study we have examined safety and efficiency of this conversion in twelve hospitalised asthmatic patients who were given the new sustained-release theophylline pellets formulation for once-daily evening administration. The lung function parameters (FEV1, VC, RV, and Rt) and serum theophylline concentrations were monitored. So, the values obtained for the last day of i.v. therapy and the fifth day of p.o. therapy were compared. We found that 75% of the patients had no change or improved lung function on the conversion. Our results indicate that this conversion from i.v. to p.o. theophylline therapy is safe and could be efficacious. Also, the maximum theophylline serum levels could safely be predicted by measuring only one serum concentration in p.o. therapy with sustained-release theophylline pellets formulation for once-daily evening administration.
Citations
Downloads
Published
How to Cite
Accepted 2018-05-06
Published 2002-02-20









